Bosch-Boonstra-Schaaf Optic Atrophy Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Bosch-Boonstra-Schaaf optic atrophy syndrome (BBSOAS) is a rare, autosomal dominant disorder that presents in early childhood.[1] In 2014 Bosch et al first described six individuals with cortical visual impairment or optic nerve abnormalities who had a mutation in the nuclear receptor subfamily 2, group F, member 1 (NR2F1) gene, also known as COUP-TF1.[2]
The most common features of BBSOAS include intellectual disability, developmental delay, and visual impairment.[1] Optic nerve pathology is the most classic finding on ophthalmologic exam.
As of 2022, there are roughly 200 known cases worldwide.[3] Currently there are no treatments for BBSOAS. Management focuses on regular comprehensive eye exams, comprehensive therapies, behavioral education, and symptom management.
Etiology
BBSOAS has autosomal dominant inheritance. It is caused by a heterozygous mutation in the NR2F1 gene ( also known as COUP-TF1) located on chromosome 5 (5q15).[1][2] This is thought to cause loss of function of this protein and dysregulation of neurodevelopment.[2]
Risk Factors
This syndrome has autosomal dominant inheritance, so having parents with NR2F1 mutations is a risk factor. However, most reported cases are from de novo mutations.[4]
Pathophysiology
BBSOAS originates from mutations in the NR2F1 gene that encodes an orphan nuclear receptor protein.[1] Studies have reported missense mutations, nonsense mutations, frameshifting insertions/deletions, non-frameshifting insertions/deletions, translation initiation variants, and whole-gene deletions.[1][2][4][5][6][7][8][9][10] There is evidence that missense mutations have a more severe phenotype than deletions.[1] Other reports have shown that mutations in the DNA-binding domain are associated with a higher prevalence of seizures, touch sensitivity, motor delay, inability to walk unassisted, and inability to communicate verbally.[11] Mouse models have shown NR2F1 is involved in cell-fate determination, differentiation, migration, and survival as well as playing a role in organogenesis. They have also shown it is mainly expressed in the optic nerve, thalamus, and cortex. This explains its specific role in cortical patterning, guidance of thalamocortical axons, and eye and optic nerve development.[2] Therefore, the current hypothesis is that mutations in NR2F1 cause loss of function of the protein, which disrupts neurodevelopment.
Diagnosis
BBSOAS is typically identified by a characteristic set of signs and symptoms and then confirmed through genetic testing. Characteristic findings include intellectual disability, developmental delay, and visual impairment. It is typically identified in childhood but can also be diagnosed in older individuals who may have been initially misdiagnosed prior to the description of BBSOAS in 2014.[1][7]
Systemic Findings
The phenotype of BBSOAS is highly variable. The most common characteristics include intellectual disability, developmental delay, and visual impairment. Other common findings include autism spectrum disorder, epilepsy, infantile spasms, hypotonia, hearing impairment, oromotor dysfunction, dysmorphic facial features, and abnormalities of the corpus callosum.[1][11] Other dysmorphic findings include protruding ears, high nasal bridge, upturned nose, epicanthal folds, upslanting palpebral fissures, and tapering fingers[12]. Patients are commonly reported to have a love of music, good long-term memory, high pain tolerance, and difficulty sleeping.[11]
Ophthalmologic Findings
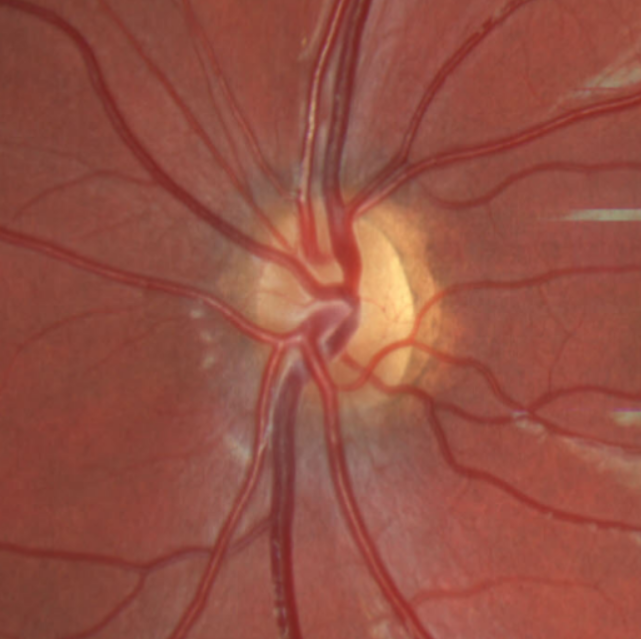
On ophthalmic examination there is typically evidence of optic atrophy with pale optic discs. There can also be excavation or hypoplasia of the optic discs.[2] Patients also commonly present with nystagmus, cortical visual impairment, and alacrima.[11]
Diagnostic procedures
Definitive diagnosis is made through full exome sequencing or NR2F1 specific sequencing.[1][2] There is one case of amniocentesis with fetal karyotyping and CNV-seq confirming an NR2F1 gene deletion in a fetus with enlarged ventricles.[4] Optical coherence tomography is typically performed to evaluate the optic nerve if the patient is able to cooperate. As children get older, they may have visual field testing performed, though this may be limited by the presence of developmental disability or nystagmus. Neuroimaging can show abnormalities of the corpus callosum including thickening or thinning, bilateral optic nerve volume loss, hypoplastic lacrimal glands, and dysgyria of the temporal lobes and perisylvian cortex.[13]
Differential diagnosis
One of the most common findings of BBSOAS is optic atrophy so a broad differential including genetic, compressive, toxic, infectious, inflammatory, and other causes should be considered. There is one report of a patient previously diagnosed with the glycosylation disorder ALG6-CDG that was later found to have BBSOAS.[7]
Management
There is no treatment for BBSOAS, but therapies can help with symptom management. Low vision specialists can be helpful for individuals with significant visual impairment. Physical, occupational, speech, and behavioral therapy can also be helpful for the developmental aspects of BBSOAS. Additionally, medical management can be beneficial if individuals develop infantile spasms or seizures. For family members of patients with BBSOAS, connecting with support groups can be helpful.
Prognosis
There is no known regression or progression of this syndrome over time. There are no reported cases of progression of optic atrophy, so the optic nerve anomalies in these patients are considered congenital and static. Most individuals will continue to require assistance and therapies throughout their life. There is no known difference in average life expectancy. BBSOAS is a newly identified syndrome and as more patients are identified, there will likely be more information on the prognosis of this condition.
Additional Resources
https://nr2f1.org/ https://www.omim.org/entry/615722
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Chen CA, Bosch DG, Cho MT, et al. The expanding clinical phenotype of Bosch-Boonstra-Schaaf optic atrophy syndrome: 20 new cases and possible genotype-phenotype correlations [published correction appears in Genet Med. 2017 Aug;19(8):962]. Genet Med. 2016;18(11):1143-1150. doi:10.1038/gim.2016.18
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Bosch DG, Boonstra FN, Gonzaga-Jauregui C, et al. NR2F1 mutations cause optic atrophy with intellectual disability. Am J Hum Genet. 2014;94(2):303-309. doi:10.1016/j.ajhg.2014.01.002
- ↑ Monnier C. BBSOAS guides/Las Guías BBSOAS. NR2F1 Foundation. https://nr2f1.org/bbsoas-guides-las-guias-bbsoas/.
- ↑ Martín-Hernández E, Rodríguez-García ME, Chen CA, et al. Mitochondrial involvement in a Bosch-Boonstra-Schaaf optic atrophy syndrome patient with a novel de novo NR2F1 gene mutation. J Hum Genet. 2018;63(4):525-528. doi:10.1038/s10038-017-0398-3
- ↑ Park SE, Lee JS, Lee ST, Kim HY, Han SH, Han J. Targeted panel sequencing identifies a novel NR2F1 mutations in a patient with Bosch-Boonstra-Schaaf optic atrophy syndrome. Ophthalmic Genet. 2019;40(4):359-361. doi:10.1080/13816810.2019.1650074
- ↑ 7.0 7.1 7.2 Starosta RT, Tarnowski J, Vairo FPE, Raymond K, Preston G, Morava E. Bosch-Boonstra-Schaaf optic atrophy syndrome (BBSOAS) initially diagnosed as ALG6-CDG: Functional evidence for benignity of the ALG6 c.391T>C (p.Tyr131His) variant and further expanding the BBSOAS phenotype. Eur J Med Genet. 2020;63(7):103941.
- ↑ Bojanek EK, Mosconi MW, Guter S, Betancur C, Macmillan C, Cook EH. Clinical and neurocognitive issues associated with Bosch-Boonstra-Schaaf optic atrophy syndrome: A case study. Am J Med Genet A. 2020;182(1):213-218. doi:10.1002/ajmg.a.61409
- ↑ Walsh S, Gösswein SS, Rump A, et al. Novel dominant-negative NR2F1 frameshift mutation and a phenotypic expansion of the Bosch-Boonstra-Schaaf optic atrophy syndrome. Eur J Med Genet. 2020;63(10):104019. doi:10.1016/j.ejmg.2020.104019
- ↑ Hobbs MM, Wolters WC, Rayapati AO. Bosch-Boonstra-Schaaf Optic Atrophy Syndrome Presenting as New-Onset Psychosis in a 32-Year-Old Man: A Case Report and Literature Review. J Psychiatr Pract. 2020;26(1):58-62. doi:10.1097/PRA.0000000000000440
- ↑ 11.0 11.1 11.2 11.3 Rech ME, McCarthy JM, Chen CA, et al. Phenotypic expansion of Bosch-Boonstra-Schaaf optic atrophy syndrome and further evidence for genotype-phenotype correlations. Am J Med Genet A. 2020;182(6):1426-1437. doi:10.1002/ajmg.a.61580
- ↑ Levin, AV, Enzenauer RW. The Eye in Pediatric Systemic Disease. Cham, Switzerland: Springer International Publishing; 2017.
- ↑ Desai NK, Kralik SF, Edmond JC, et al. Common Neuroimaging Findings in Bosch-Boonstra-Schaaf Optic Atrophy Syndrome. AJNR Am J Neuroradiol. 2023;44(2):212-217. doi:10.3174/ajnr.A7758