Blepharophimosis Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Blepharophimosis-Ptosis-Epicanthus Inversus Syndrome (BPES) is an inherited eyelid syndrome presenting with telecanthus, epicanthus inversus, and ptosis.
Disease Entity
Oculoplastics, Pediatric Ophthalmology
Disease
It is an uncommon inherited dysmorphic syndrome, which primarily affects the soft tissues of the midface, with the following signs:
- Blepharophimosis
- Ptosis
- Epicanthus inversus
- Telecanthus
- Ectropion
- Strabismus
- High arched brows
- Lopped ears
Two types:
- Type 1 : associated with primary ovarian failure
- Type 2: no systemic association
History
Familial cases of the classic eyelid malformations have been described by Vignes and Ammon since the mid 1800s.[1]
Definitions
BPES is a clinical syndrome that involves blepharophimosis, ptosis, epicanthus inversus, and telecanthus.
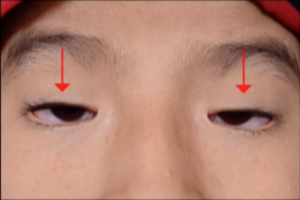
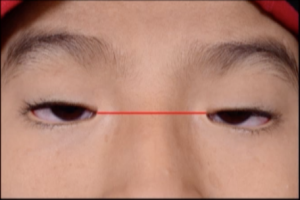
Horizontal shortening of palpebral fissure: Normal horizontal palpebral fissures measure 25-30mm; in BPES, the palpebral fissures measure 20-22mm (see red lines below).
Ptosis: Upper eyelid droopiness resulting in vertical narrowing of palpebral fissures (see below). BPES involves dysplasia of bilateral levator palpebrae superioris muscles, resulting in poor levator function and bilateral ptosis.[2]
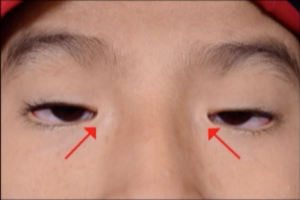
Epicanthus Inversus: Skin fold arising from the medial lower eyelid and ascending to the upper eyelid (see below). Both the plica semilunaris and caruncle may appear hypoplastic.[3] Epicanthus inversus has high specificity for BPES and occurs bilaterally.
Telecanthus: Increased distance between the medial canthi.[4][5]
Images obtained from multimedia presentation by Abhilasha Maheshwari, MBBS. © 2017 American Academy of Ophthalmology. Clinical and Surgical Videos. https://www.aao.org/clinical-video/surgical-management-of-blepharophimosis. Accessed July 13, 2019.
Etiology
BPES is a genetic syndrome inherited in an autosomal dominant fashion. Up to fifty percent of cases are thought to be sporadic from de novo mutations in the FOXL2 gene. BPES has an unknown prevalence.
Pathophysiology
The phenotype of BPES can be traced back to dysfunction or absence of the FOXL2 gene product. FOXL2 is a single gene exon expressed in the mesenchyme of developing eyelids and ovaries. It is the only known gene directly implicated in the BPES phenotype. FOXL2 codes for a transcription factor that regulates the expression of various other genes, including those involved in inflammation, transcription, proteolysis, apoptosis, and detoxification of reactive oxygen species.[6]
FOXL2 expression can be altered by intragenic mutations, larger genomic deletions involving the FOXL2 gene, and even genomic alterations outside of the FOXL2 locus. Intragenic mutations account for about 72% of mutations in BPES and can manifest as nonsense, frameshift, missense, or in-frame mutations. Nonsense and frameshift mutations severely impair gene function, but the effect of missense mutations can vary. The location of the missense mutation plays a role in its pathogenic potential. Mutations in the forkhead domain, the region of the protein that binds to DNA, are likely to be pathogenic. In-frame mutations tend to manifest as poly-alanine expansions. These expansions are caused by slippage of DNA polymerase when duplicating trinucleotide repeats and account for about 31% of all intragenic mutations in BPES overall.[7][8] Large genomic deletions involving FOXL2 region account for about 10% of the genetic defects in BPES.[9] Genomic alterations in loci outside of the FOXL2 region have also been found to account for about 4% of the known genetic defects in BPES. These remote alterations affect the transcription of FOXL2 by impacting distant regulatory elements.
Patients with a pathologic FOXL2 variation nearly always express the BPES phenotype in the eyelids, while the effect on the ovary varies. The aforementioned poly-alanine expansions are more likely to be associated with BPES type two (without ovarian involvement), while truncated proteins are more likely to be associated with BPES type one (with ovarian involvement). Importantly, however, even some patients with poly-alanine expansions demonstrated some varying degrees of ovarian dysfunction. This could suggest an overlap between the two types of BPES, with the ovarian involvement being milder or later onset in type two.[10][11] Clinical follow-up is therefore crucial for accurate evaluation of ovarian function. The role of FOXL2 in the pathogenesis of BPES is therefore well established, but further investigation is required to delineate a more precise relationship between genotype and phenotype.[7]
Diagnosis
The diagnosis is mostly made by the presence of clinical features which include:
- Blepharophimosis (horizontal shortening of palpebral fissure)
- Ptosis (droopy lid)
- Epicanthus inversus (prominent skin fold extend from lower to the upper lid)
- Telecanthus (widened intercanthal distance )
Other associations include lower lid ectropion, widened nasal bridge or superior orbital rim hypoplasia, or hypertelorism, anteverted ears, and thick highly arched eyebrows.
Family history of bearing a similar appearance, diagnosis, or primary ovarian failure can help clinch the diagnosis.
Clinical Presentation
BPES presents at birth with the four cardinal eyelid malformations: blepharophimosis, ptosis, epicanthus inversus, and telecanthus (See Figure 1). Other associations include euryblepharon (a type of lower lid ectropion), widened nasal bridge, superior orbital rim hypoplasia, hypertelorism, anteverted ears, short philtrum, and thick highly arched eyebrows. A retrospective analysis of BPES patients also identified amblyopia (in 41% of patients), refractive errors (34%), strabismus (20%), and nystagmus (6%). Of the patients with strabismus, 70% showed esotropia, 25% showed exotropia, and 5% showed hypertropia.[12] The premature ovarian failure in BPES type I presents as normal menarche followed by loss of menses before the age of forty. In addition to decreased fertility, this also causes other symptoms associated with hypoestrogenism including, but not limited to hot flashes, decreased libido, vaginal dryness, and reduced bone density. As this is autosomal dominant, patients may also report a family history of similar appearance, diagnosis, or premature ovarian failure.
Laboratory test
Diagnosis of BPES is mainly clinical. Gene alterations in FOXL2 or cytogenetic rearrangements involving chromosome 3 can be identified using molecular genetic testing and chromosomal analysis, respectively. This can be useful for genetic counseling and/or diagnostic confirmation. Female patients can also undergo laboratory evaluation of ovarian function with serum levels of FSH, LH, estradiol, and progesterone. Pelvic ultrasound may reveal a hypoplastic uterus.
Differential diagnosis
- Hereditary Congenital Ptosis 1
- Hereditary Congenital Ptosis 2
- Ohdo Blepharophimosis Syndrome
- Michels Syndrome
- Ptosis with External Ophthalmoplegia
- Noonan Syndrome
- Marden-Walker Syndrome
- Schwartz-Jampel Syndrome
- Dubowitz Syndrome
- Smith-Lemli-Opitz Syndrome
- KANSL1-related intellectual disability syndrome
Management
Management of BPES should address the eyelid malformations, any related visual impairment, and primary ovarian insufficiency.
Prompt surgical correction of the eyelid malformations can help prevent visual impairment from amblyopia, but later surgeries allow for more precise ptosis correction. For this reason, the exact timing of eyelid surgery is controversial. Once amblyopia has been ruled out, management of the eyelid malformations typically involves correcting the epicanthus inversus and telecanthus at three to five years of age, followed by ptosis correction one year later. A medial canthoplasty is typically done to target the epicanthus inversus and telecanthus, and a lateral canthoplasty can widen the horizontal palpebral fissure and help correct the blepharophimosis.
Below are general recommendations for surgical intervention in BPES:
- surgical correction can be delayed until the patient is 3 to 5 years of age, if the visual axis remains unobstructed.
- a single-stage or two-stage correction may be offered if the visual axis is deemed obstructed but the vertical interpalpebral fissure height measures more than 2 mm.[13]
- a two-stage correction, beginning with ptosis correction first to avoid the development of vision-deprivation amblyopia, later followed by a medial canthoplasty, may be considered early if the central visual axis is deemed obstructed and the vertical interpalpebral fissure height measures less than 2 mm.[14]
Epicanthus fold and telecanthus :
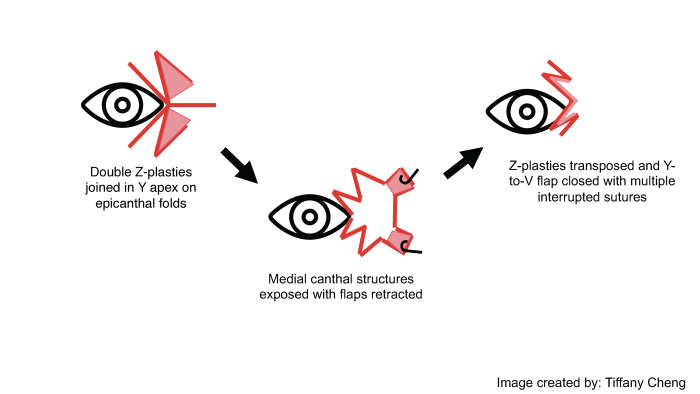
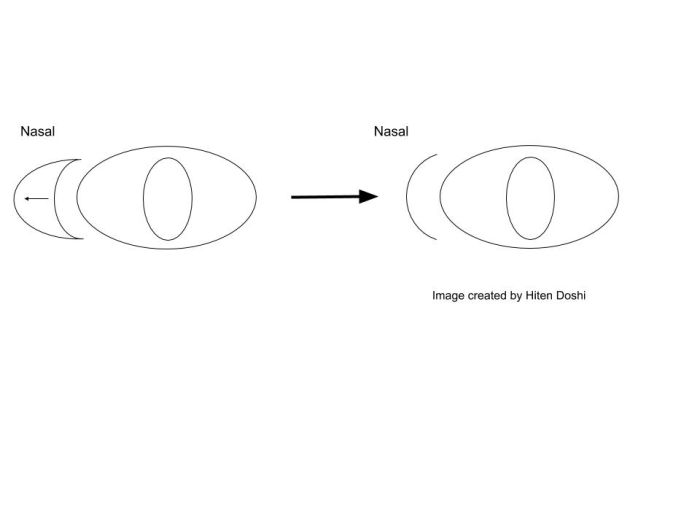
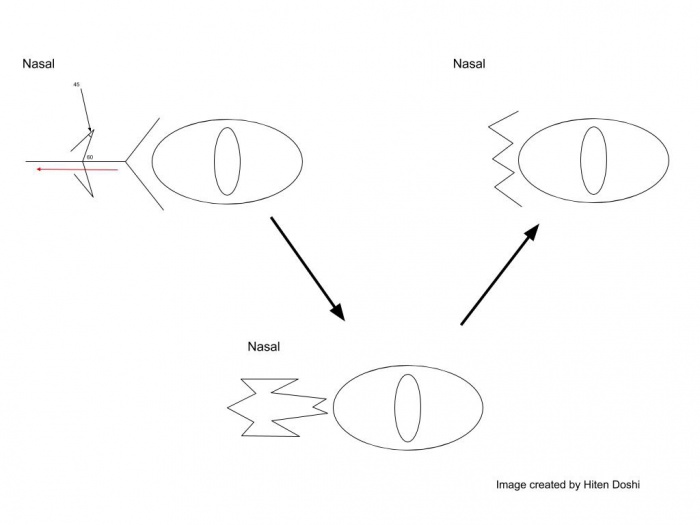
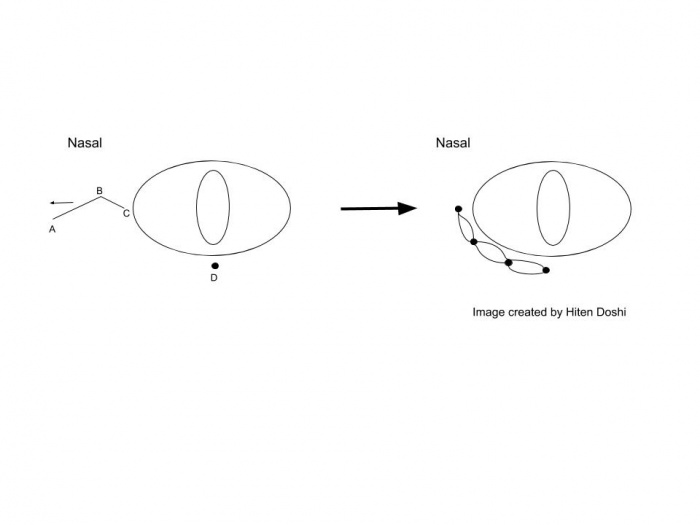
Surgical approach for the medial canthoplasty has varied. Options include the Y to V flap, the Five-Flap technique (combination of Y-to-Z flap with double opposing Z-plasties on the apex), the Hornblast C-to-U flap (see video), and Mustarde’s technique. Shown below are Y to V flap, the Five-Flap technique, Hornblast C-to-U flap, and Mustarde’s technique:
Y to V flap:
Five Flap:
Hornblast C-to-U flap:
Mustarde’s technique:
These are all viable options, but have been criticized for their postoperative scarring or, in the case of Mustarde’s technique, cumbersome mapping, measurements, and complexity.[15][16][17][18][19][20] A new surgical approach has been described that involves a modified skin redraping method. This method avoids undesirable scarring near the medial canthus by transferring tension from the medial canthus to the subciliary incision. While Mustarde’s technique has been previously popular, this new surgical technique could be a viable alternative with potentially better cosmetic outcomes.[15] Telecanthus can be corrected by shortening the medial canthal tendon and fixing it to subcutaneous tissue.[15] Transnasal wiring can also be used.[21] An image demonstrating the modified skin redraping method is shown below, see the original publication for full details[15]:
Ptosis:
The ptosis in BPES has generally been corrected separately, and tends to be severe due to poor levator function. Frontal flap suspension has been used, but may result in underdevelopment of the frontalis muscle.[22] Supramaximal levator resection has been shown to result in both successful ptosis correction and improvement in levator function.[22]
Primary ovarian falure:
Patients with BPES should also undergo genetic evaluation and counseling. Females should be referred to an endocrinologist or gynecologist to assess for primary ovarian insufficiency. Premature ovarian insufficiency in BPES can be managed similarly to other etiologies; hormone replacement therapy can be used to manage symptoms of hypoestrogenism. This is especially useful in young patients to minimize post-menopausal symptoms and risk of osteoporosis. Patients with premature ovarian insufficiency, who desire children, should be counseled about their options including cryopreservation of ovarian tissue, oocyte donation, and adoption.[23]
BPES Video: [[1]]
Prognosis
Excellent eyelid surgery results have been published, typically requiring multiple staged surgeries.
Additional Resources
- American Academy of Ophthalmology. Surgical Management of Blepharophimosis. https://www.aao.org/clinical-video/surgical-management-of-blepharophimosis San Francisco: American Academy of Ophthalmology, 2020. Accessed June 08, 2020.
References
- ↑ Vignes A. Epicanthus hereditaire. Rev Gen Ophthalmol (Paris) 1889;8:438–439
- ↑ Beaconsfield M, Walker JW, Collin JR. Visual development in the blepharophimosis syndrome. Br J Ophthalmol 1991;75:746–748.
- ↑ Kohn R, Romano PE. Blepharoptosis, blepharophimosis, epicanthus inversus, and telecanthus--a syndrome with no name. Am J Ophthalmol. 1971 Sep;72(3):625-32. doi: 10.1016/0002-9394(71)90864-6. PMID: 5568616.
- ↑ Verdin H, De Baere E. Blepharophimosis, Ptosis, and Epicanthus Inversus. 2004 Jul 8 [Updated 2015 Feb 5]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews ® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019.
- ↑ Tyers, A. G. (2011). "The blepharophimosis-ptosis-epicanthus inversus syndrome (BPES)." Orbit 30(5): 199-201.
- ↑ Batista F Vaiman D, Dausset J, Fellous M Veitia R. 2007. Potential targets of FOXL2 a transcription factor involved in craniofacial and follicular development identified by transcriptomics. Proc Natl Acad Sci USA 104:3330-3335
- ↑ 7.0 7.1 Beysen, D., et al. (2009). "FOXL2 mutations and genomic rearrangements in BPES." Hum Mutat 30(2): 158-169.
- ↑ Brown LY, Brown SA. 2004. Alanine tracts: the expanding story of human illness and trinucleotide repeats. Trends Genet 20:51-58
- ↑ Beysen D, Raes J, Leroy BP, Lucassen A, Yates JR, Clayton-Smith J, Ilyina H, Brooks SS, Christin-Maitre S, Fellous M, Fryns JP, Kim JR, Lapunzina P, Lemyre E, Meire F, Messiaen LM, Oley C, Splitt M, Thomson J, Van de Peer Y, Veitia RA, De Paepe A, De Baere E. 2005. Deletions involving long-range conserved nongenic sequences upstream and downstream of FOXL2 as a novel disease causing mechanism in blepharophimosis syndrome. Am J Hum Genet 77:205–218.
- ↑ Crisponi, L., Deiana, M., Loi, A., Chiappe, F., Uda, M., Amati, P., Bisceglia, L., Zelante, L., Nagaraja, R., Porcu, S. et al. (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/ epicanthus inversus syndrome. Nature Genet., 27, 159–166.
- ↑ De Baere, E., Dixon, M.J., Small, K.W., Jabs, E.W., Leroy, B.P., Devriendt, K., Gillerot, Y., Mortier, G., Meire, F., Van Maldergem, L. et al. (2001) Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype-phenotype correlation. Hum. Mol. Genet., 10, 1591–1600.
- ↑ Dawson EL, Hardy TG, Collin JR, Lee JP. The incidence of strabismus and refractive error in patients with blepharophimosis, ptosis and epicanthus inversus syndrome (BPES). Strabismus. 2003;11:173–7. PubMed
- ↑ Wu SY, Ma L, Tsai YJ, Kuo JZ. One-stage correction for blepharophimosis syndrome. Eye (Lond). 2008 Mar;22(3):380-8. doi: 10.1038/sj.eye.6702644. Epub 2006 Nov 17. PMID: 17115018.
- ↑ Beckingsale PS, Sullivan TJ, Wong VA, Oley C. Blepharophimosis: a recommendation for early surgery in patients with severe ptosis. Clin Exp Ophthalmol. 2003 Apr;31(2):138-42. doi: 10.1046/j.1442-9071.2003.00621.x. PMID: 12648048.
- ↑ 15.0 15.1 15.2 15.3 Sa HS, Lee JH, Woo KI, Kim YD. A new method of medial epicanthoplasty for patients with blepharophimosis ptosis-epicanthus inversus syndrome. Ophthalmology. 2012;119:2402–7. PubMed
- ↑ Blair VP, Brown JB, Hamm WG. Correction of ptosis and of epicanthus. Arch Ophthalmol 1932;7:831–46.
- ↑ Johnson CC. Surgical repair of the syndrome of epicanthus inversus, blepharophimosis and ptosis. Arch Ophthalmol 1964;71:510–6.
- ↑ Anderson RL, Nowinski TS. The five-flap technique for blepharophimosis. Arch Ophthalmol 1989;107:448–52.
- ↑ Mustardé JC. Epicanthus and telecanthus. Br J Plast Surg 1963;16:346–56.
- ↑ Anderson RL, Nowinski TS. The Five-Flap Technique for Blepharophimosis. Arch Ophthalmol.1989;107(3):448–452. doi:10.1001/archopht.1989.01070010458045
- ↑ Mustardé JC. Epicanthus, telecanthus, blepharophimosis and related conditions. In: Mustardé JC, ed. Repair and Reconstruction in the Orbital Region: A Practical Guide. 2nd ed. Edinburgh: Churchill Livingstone; 1980:346–51.
- ↑ 22.0 22.1 Decock CE, Shah AD, Delaey C, Forsyth R, Bauters W, Kestelyn P, De Baere E, Claerhout I. Increased levator muscle function by supramaximal resection in patients with blepharophimosis-ptosis-epicanthus inversus syndrome. Arch Ophthalmol. 2011b;129:1018–22.
- ↑ Goswami D, Conway GS. 2005. Premature ovarian failure. Hum Reprod Update 11:391–410.
- 2. Ammon FAv. Klinische darstellung der krankheiten undbildungsfehler des menschlichen auges, der augenglides und der thranewerkzeuge. G Reimers, Berlin; 1841.
- 3. Duane, T. D., Jaeger, E. A., & Tasman, W. (1992). Chater 59, Ocular Genetics. In Duane's Foundations of Clinical Ophthalmology (Vol. 3). Harper & Row.
- 10. De Baere E, Lemercier B, Christin-Maitre S, Durval D, Messiaen L, Fellous M, Veitia R. 2002. FOXL2 mutation screening in a large panel of POF patients and XX males. J Med Genet 39:e43.
- 11. De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, Devriendt K, Dixon M, Fellous M, Fryns JP, Garza A, Jonsrud C, Koivisto PA, Krause A, Leroy BP, Meire F, Plomp A, Van Maldergem L, De Paepe A, Veitia R, Messiaen L. 2003. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype–phenotype correlation. Am J Hum Genet 72:478–487.
- 15. Harris, S. E., et al. (2002). "Identification of novel mutations in FOXL2 associated with premature ovarian failure." Mol Hum Reprod 8(8): 729-733.
- 16. Blepharophimosis, Ptosis, Epicanthus Inversus Syndrome. NORD (National Organization for Rare Disorders). https://rarediseases.org/rare-diseases/blepharophimosis-ptosis-epicanthus-inversus-syndrome/. Accessed July 7, 2019.