Biosimilars in Ophthalmology
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
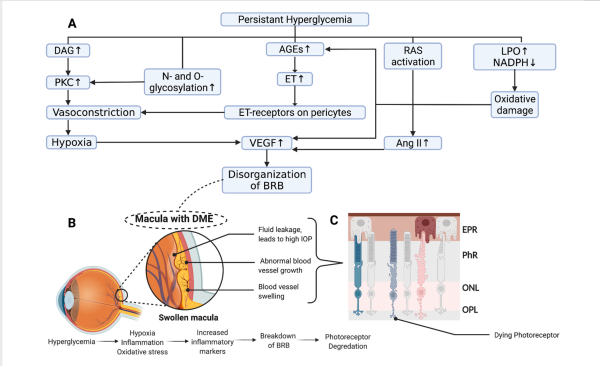
Neovascular Age Related Macular Degeneration (nAMD) and Diabetic Macular Edema (DME) are leading causes of severe vision loss in the general population. The prevalence of these conditions is expected to increase as the number of people with diabetes rises and the general population ages. A key player in both conditions is Vascular Endothelial Growth Factor (VEGF), a signal protein that promotes the growth of abnormal blood vessels and increases vascular permeability (Figure 1).[1][2][3] Anti-VEGF therapies such as ranibizumab (RZB) (Lucentis), aflibercept (Eylea), and faricimab-svoa (Vabysmo) have been developed to inhibit VEGF's actions, thereby slowing the progression of nAMD and DME.[4][5]
Despite the efficacy of these treatments, the high cost of ranibizumab, aflibercept, and faricimab-svoa is a significant disadvantage. Biosimilars are now being offered as a potentially more affordable alternative treatment for nAMD and DME. The safety and efficacy of few biosimilars have been proved in evidence-based literature.[6][7] Their lower cost may present advantages to patients, physicians, payers, and health care systems. With better education on biosimilars, these treatments have the potential to reduce costs, increase treatment adherence, and improve patient outcomes.[8]
Another Anti-VEGF therapy, bevacizumab (Avastin), is an alternative option available via compounding pharmacies. Initially approved to treat different types of cancer, Avastin has found significant off-label use in the treatment of nAMD and DME, given its low cost. The FDA permits this off-label use due to numerous studies demonstrating Avastin's efficacy. There have been instances of cluster endophthalmitis after use of intravitreal Avastin, which were mostly related to spurious drug or noncompliance to standard sterile compounding procedures.[9][10] However, the development of biosimilars to bevacizumab raises a potential legal issue that might result in the prohibition of Avastin's off-label use. This scenario could, in turn, lead to an increase in overall patient costs.[11]
This article aims to summarize the current literature about biosimilars and their safety, efficacy, and quality.
Biosimilars in Ophthalmology
An Overview
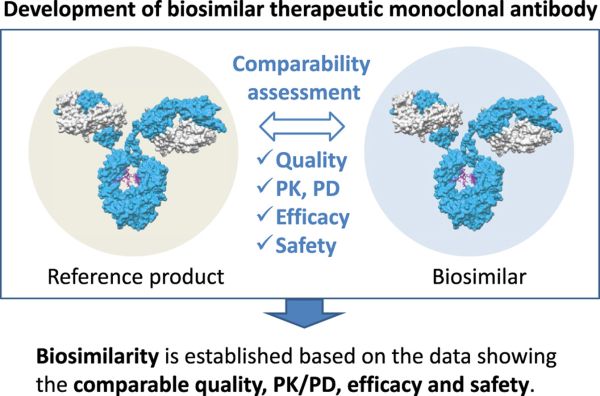
As the patents for original biologics used to treat nAMD and DME approach expiration, numerous companies are striving to replicate these biologics. Biosimilars are drugs that are similar to reference drugs and mimic their effects, but they do not have the same identical active ingredients.[13] Unlike small molecule drugs, biologics and their biosimilars are large, complex molecules (Figure 2).[12] Furthermore, the "formula" for developing a biosimilar is not provided by the originator biologics. Consequently, companies often have limited information about the original biologic and must rely on techniques such as reverse engineering to develop biosimilars.[14]
Companies’ motivation to develop biosimilars is multifaceted. The development of original biologics is a lengthy and costly process, typically spanning 10-15 years and costing an average of 1.2-2.5 billion dollars. In contrast, biosimilars can be manufactured within 8-10 years at approximately a tenth of the cost.[15][16] A significant portion of the cost and time savings in developing biosimilars is due to their unique approval process, which requires fewer clinical trials and places a greater emphasis on demonstrating analytical bioequivalence to prove similarity to the reference biologic.[14]
A critical stage in the development of biosimilars involves the selection of a reference product and the subsequent development and cultivation of a cell line.
- This process begins with the identification of the relevant gene that encodes the target protein of the reference product , which is then cloned into a vector.
- The vector is subsequently transfected into host cells, typically Chinese hamster ovary cells. These transfected cells are then selected for stable expression of the protein of interest.
- Finally, the cells are amplified to create a cell bank, which serves as the foundation for biosimilar production.[18]
The development of biosimilars is complex and differs from that of generic drugs, necessitating a clear differentiation and understanding of their approval process. While generic drugs require matching the chemical formula and synthesis, biosimilars require live cells. Additionally, due to the fact that biosimilars are not exact copies in terms of formulation, there is a need for increased attention to assessing stability and immunogenicity.[14] Only minor differences in clinically inactive components, known as excipients, are allowed in biosimilars.[19]
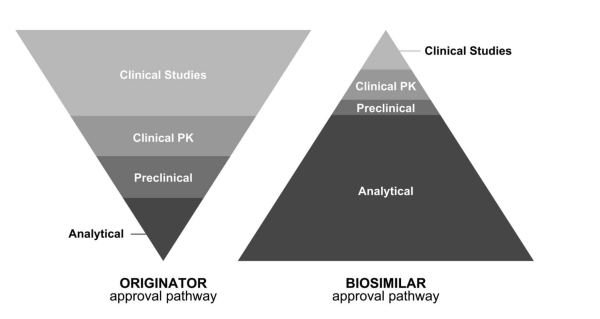
The United States (US) established safety and bioequivalence standards for biosimilars in 2009. For approval, the US Food and Drug Administration (FDA) requires evidence that there are “no clinically meaningful differences in safety, purity, and potency between the biosimilar and reference product”.[20] This is achieved via a stepwise approach, which differs from the drug approval pathway for the reference biologic, as biosimilar approval relies more heavily on analytical data performed to establish similarity in physicochemical properties (Figure 3).[8][17] The efficacy of a biosimilar product is assessed based on pre-determined equivalence margins using selected clinical endpoints.[8] Following assessment by regulatory authorities such as the FDA, if no clinically meaningful differences are found, the biosimilar is automatically approved for all indications of the reference drug, even if the biosimilar was not directly studied in clinical trials. This principle, referred to as “indication extrapolation,” reduces the cost of manufacturing biosimilars.[20]
A Closer Look
This section offers an in-depth review of biosimilars, both approved and in development, for the treatment of nAMD and DME. The reference drugs currently used for these conditions are RBZ (Lucentis), and aflibercept (Eylea). Due to the nature of the biosimilar approval process, most clinical trials primarily focus on evaluating safety, efficacy, pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity in patients with nAMD. Additionally, we will discuss other biosimilars that are in the advanced stages of clinical trials.
FDA (Food and Drug Administration, USA) approved
Byooviz, ranibizumab-nuna
The first FDA-approved biosimilar to RBZ is Byooviz (SB-11), a product gaining its approval status in Septmeber 2021.[21] A Phase 3 study of SB11 was a randomized, double-masked, multicenter study evaluating the efficacy, safety, PK, and immunogenicity of SB11. The study had a primary endpoint of change from baseline in best-corrected visual acuity (BCVA) at week 8 and central subfield thickness (CST) at week 4. The secondary endpoints included PK, safety, long-term efficacy, and immunogenicity.[22] [23] The study began with 705 patients with nAMD who were randomized to receive either SB11 or reference RZB in monthly injections (0.5 mg), and 634 patients continued to receive treatment for the entire study length of 48 weeks. The primary endpoint was met as the adjusted treatment difference between SB11 and RBZ in BCVA was -0.8 letters (90% confidence interval (CI) -1.8 to 0.2) and the change from baseline in CST was -8.4 μm (95% CI -19.4 to 2.7). In a post-hoc analysis of this study, one-year efficacy outcomes in nAMD were determined, the study found that baseline factors such as age, gender, and baseline visual acuity were not associated with the treatment response to both SB11 and the reference RZB, based on the primary endpoint of a previous study.[24]
Cimerli, ranbizumab-eqrn
FYB-201, another biosimilar to RZB, received interchangeable product (IP) status for all five indications of reference drug RZB in August 2022. The COLUMBUS-AMD clinical trial, a multicenter, evaluation-masked, parallel-group, 48-week, Phase 3 randomized study, was conducted to evaluate FYB-201 for the treatment of nAMD-associated macular edema. This study involved 477 patients with nAMD. Both the FYB-201 and reference RBZ groups demonstrated an improvement in BCVA throughout the study period. Specifically, by week 48, the mean change from baseline was +7.8 ± 11.7 (median 8.0) for FYB201 and +8.0 ± 11.3 (median 8.0) ETDRS letters for reference RBZ. The ANCOVA least squares mean difference for the change from baseline in BCVA between FYB201 and reference RBZ at week 48 was a negligible -0.1 ETDRS letters, with a 90% CI of -1.8 to 1.7 (p>0.5). The study also reported that there was no evidence of increased side effects, elevated intraocular pressure, or signs of ocular toxicity observed in the study.[25]
FDA-approved interchangable biosimilars to Eylea (Aflibercept)
- Yesafili (aflibercept-jbvf) and
- Opuviz (aflibercept-yszy)
Awaiting Approval in the United States
CKD-701
The CKD-701, a biosimilar candidate to RZB, was evaluated in a Phase 3 clinical trial for the treatment of nAMD. The study included 291 participants with nAMD and was a randomized, double-blind, parallel group, comparative multicenter trial that evaluated the efficacy, safety, PK properties, and immunogenicity of CKD-701 compared to the reference drug, RZB. The primary efficacy endpoint was the proportion of patients who lost less than 15 letters in BCVA from the baseline at month 12. The proportion of patients who lost less than 15 letters in BCVA from the baseline at 3, 6, and 12 months were 97.95%, 97.92%, and 95.52% respectively for CKD-701, and 98.62%, 97.90%, and 95.65%, respectively for RZB (P>0.05). Secondary efficacy endpoints included the proportion of patients who gained 15 or more letters in BCVA from the baseline at month 12, and the mean change in BCVA from the baseline at month 12. The proportion of patients who gained 15 or more letters in BCVA from the baseline at 3, 6, and 12 months were 16.44%, 21.53%, and 24.63%, respectively for CKD-701, and 17.24%, 19.58%, and 21.01% respectively for RZB. Again, the differences were not statistically significant (all P>0.05). The study concluded that CKD-701 has comparable efficacy and safety profiles to RZB, suggesting that CKD-701 may be considered a viable alternative to the reference innovator product in patients with nAMD.[26]
XSB-001
Xbrane Biopharma has developed a biosimilar to RBZ, known as XSB-001, which has undergone evaluation in a Phase 3, multicenter, randomized, double-masked, parallel-group clinical trial.[27] This trial aimed to assess the efficacy, safety, and immunogenicity of XSB-001 compared to the reference product, RBZ, for the treatment of nAMD. The least squares mean treatment difference was -1.8 ETDRS letters (90% CI, -2.9 to -0.7; 95% CI, -3.1 to -0.5) at week 8, and -1.5 ETDRS letters (90% CI, -3.3 to 0.4; 95% CI, -3.6 to 0.7) at week 52. No clinically significant differences were observed between the treatments in terms of anatomical, safety, or immunogenicity endpoints through week 52. The study concluded that XSB-001 demonstrated biosimilarity to the reference product, RBZ, in patients with nAMD. The 52-week treatment with XSB-001 was generally safe and well-tolerated, exhibiting a safety profile similar to that of the reference product.[28]
Xlucane
In 2021, Xbrane Biopharma AB announced that halfway through the phase 3 equivalence trial with RXC Xlucane met the primary endpoint demonstrating equivalent efficacy in change of BCVA at week 8 of treatment compared to RBZ.[29] Xbrane Biopharma announced plans to submit the necessary documents to the FDA in 2021, however, to date the National Library of Medicine states “the number of participants at risk appears inconsistent with data here or in other parts of the record.”[30]
Approved Outside of the United States
Razumab
Razumab, the first biosimilar to RBZ, was approved and introduced to the market in India in 2015.[31] However, in 2019, 31 reports of ocular inflammation led the Vitreo Retina Society of India to issue an advisory against a portion of the circulating Razumab supply.[32] This prompted several clinical trials to assess the safety, efficacy, and immunogenicity of Razumab. A retrospective observational study was conducted on patients with nAMD, who were treated with either RBZ or Razumab. The study found that the baseline BCVA was 0.57±0.27 logMAR in the Razumab group and 0.61±0.25 in the RBZ group. At 3, 6, 9, and 12 months, BCVA improved significantly in both groups (P<0.0001). Similarly, the baseline CMT was 420.39 ±54.45 μm in the Razumab group and 407.82±53.07 μm in the RBZ group. At 3, 6, 9, and 12 months, CMT decreased significantly in both groups (P<0.0001). These findings suggest that Razumab has comparable efficacy to RBZ in the treatment of nAMD, despite the initial concerns about ocular inflammation.[33]
LUBT010
LUBT010, a biosimilar to RZB, was developed in India and evaluated in a prospective, double-blind, multicenter phase three study across 19 centers in the country. The study compared the efficacy, safety, and immunogenicity of LUBT010 to RBZ. The difference between LUBT010 product and RBZ for the proportion of patients who lost less than 15 letters was within the equivalence margin (intention-to-treat population: 1.0%; 95% (CI), -3.3% to 5.4% and per protocol population: 1.2%; 95% CI, -3.2% to 6.4%). The incidence of treatment-emergent adverse events was comparable, with 11 (10.89%) patients in the LUBT010 group and 19 (18.81%) patients in the RBZ group experiencing at least one treatment-emergent adverse event. The immunogenicity incidence, as assessed by the proportion of patients with positive anti-drug antibodies, was numerically lower in LUBT010 (4.95%) than in RBZ (12.87%). The study concluded that LUBT010 demonstrated therapeutic equivalence, desirable safety, and a favorable immunogenicity profile compared to RBZ.[34]
Aflibercept biosimilars
Awaiting Approval in the United States
SB15
SB15 is the only biosimilar with a completed phase 3 randomized clinical trial assessing equivalent BCVA outcomes and safety profile to reference Aflibercept in patients with nAMD. This randomized double-masked study found of 449 participants the least squares mean change in BCVA from baseline to week 8 in the SB15 group was equivalent to that in the Aflibercept group (6.7 letters vs 6.6 letters, respectively, 95% CI, -1.3 to 1.4). Comparable efficacy between treatment groups was maintained up to week 32 (least squares mean change from baseline in BCVA: SB15, 7.6 letters vs. Aflibercept, 6.5 letters; least squares mean change from baseline in central subfield thickness: SB15, −110.4 μm vs. Aflibercept, −115.7 μm). No clinically relevant differences were observed in the incidence of treatment-emergent adverse events (TEAEs) (SB15, 107/224 [47.8%] vs Aflibercept, 98/224 [43.8%]) and ocular TEAEs in the study eye (SB15, 41/224 [18.3%] vs. Aflibercept, 28/224 [12.5%]). SB15 and Aflibercept showed equivalent efficacy and comparable safety, pharmacokinetics, and immunogenicity in participants with nAMD.[35]
Comparison of Biosimilars
Cost
Given the nature of the biosimilar approval process, data regarding cost and approved indications is currently limited to the two RZB biosimilars with FDA approval. However, it's important to note that while only SB11 (Byooviz) and FYB-201 (Cimerli) biosimilars for RZB are currently approved , a significant number are in development. The introduction of these biosimilars is expected to further reduce the cost of this essential treatment. According to Medicare Part B payments data from 2008-2021, RBZ and Aflibercept accounted for over 10% of total Medicare Part B payments, with an average cost of $1,673.59 and $1,385.95 per 0.5 mg service, respectively.[36] The current Medicare allowable payments for 0.3 mg of RBZ, 0.5 mg of RBZ, and 2 mg of Aflibercept are $775.24, $1292.04, and $1805.99, respectively.
To compare the cost of the available biosimilars, we must use the Medicare allowable payments for each reference drug and compare them to the wholesale acquisition costs (WACs) of the biosimilars, as their Medicare allowable payments are currently unavailable. As of November 12, 2022, the WACs for RZB-nuna 0.5 mg, RZB-eqrn 0.3mg, and RZB-eqrn 0.5mg are $1130, $816, and $1360, respectively.[11] The patient cost is calculated based on Medicare payment of 83.104% of the average sales price (ASP) and 21.2% of the ASP, respectively. If all patients currently on RZB 0.5 mg, RZB 0.3 mg, or Aflibercept 2 mg were switched to a less expensive biosimilar, the savings would amount to $132 million for Medicare and $33.6 million for patients.[11]
To help increase the adoption of biosimilars, ‘The Inflation Reduction Act, increases the “add-on” for certain qualifying biosimilars from 6 to 8 percent of ASP for a 5-year period starting in October of 2022. The effect of this payment policy remains to be seen, but greater ASP payment could be helpful in increasing biosimilar adoption and increasing competition in the drug market.[36]
Table 1. A cost comparison of Biosimilars to their reference drugs.
| Drug | WAC ($) | Medicare Allowable ($) | ASP ($) | Medicare
Payment ($) |
Patient Cost
($) |
|---|---|---|---|---|---|
| RZB 0.3 mg,
0.5mg |
775.24,
1292.04 |
731.36,
1218.91 |
607.79,
1012.96 |
155.05
258.41 | |
| BYOOVIZ 0.5 mg | 1130 | 1163.90 | 967.25 | 178.18 | |
| CIMERLI 0.3,
0.5 mg |
816,
1360 |
840.48,
1400.8 |
698.47,
1164.12 |
1164.12,
296.97 | |
| Aflibercept 2 mg | 1805.99 | 1703.7 | 1415.90 | 361.20 |
A research study in Japan focused on evaluating the cost-effectiveness of, RZB BS, particularly as most Japanese patients with AMD have transitioned from "as-needed" (PRN) to "treat and extend" (TAE) dosing schedules. The study employed a comprehensive cost-effectiveness analysis to assess the long-term healthcare expenses and quality-adjusted life years (QALYs) for various treatment options for neovascular AMD.
The study found that the RZB BS TAE regimen was more cost-effective, with a total cost of JPY 1,215,458 less than its brand-name equivalent, Lucentis. When compared to other treatments, the cost savings were substantial, ranging from JPY -2,075,183 to JPY -3,539,402.[37] However, it should be noted that the study was funded by the Senju Pharmaceuticals, the company that developed the biosimilar.
Indications
Table 2: FDA-Approved Indications for Various Biosimilars and Their Reference Drugs
| Drug | Indications |
| RZB | AMD, RVO, DME, DR, DR, mCNV[38] |
| CIMERLI | AMD, RVO, DME, DR, mCNV[39] |
| BYOOVIZ | AMD, RVO, mCNV[40] |
| AFLIBERCEPT | AMD, RVO, DME DR, DR, mCNV[41] |
To stimulate the development of biosimilars, the FDA employs a process known as "indication extrapolation," which helps reduce the manufacturing costs of biosimilars. According to the FDA, a biosimilar may be approved for indications or populations without direct clinical studies in those indications or populations, provided the manufacturer offers sufficient scientific justification. This justification is based on several factors, including:
• All available information in the biosimilar application
• The FDA's determination of the safety and efficacy of the reference product for the approved indications
• Knowledge and consideration of various scientific factors for each indication
Furthermore, an interchangeable biosimilar is a biosimilar that can be substituted for the reference product without the intervention of the prescribing healthcare provider. To achieve interchangeable status, the manufacturer must provide information demonstrating that the proposed product is expected to produce the same clinical result as the reference product in any given patient. This is typically accomplished through a switching study, where patients alternate between the reference product and the interchangeable biosimilar, and their results are compared to those treated solely with the reference product. Currently, Cimerli holds interchangeable status with RBZ.[42]
Future Perspectives
Table 3: Current Development Status of Aflibercept Biosimilars
| Aflibercept Biosimilars in Development | Clinical Trial | Stage |
|---|---|---|
| SB15 (Samsung Bioepis) | NCT04450329[43] | Phase 3, completed |
| M710/MYL-1701P (Mylan-Momenta) | NCT03610646[44] | Phase 3, pending approval |
| FYB203 | NCT04522167[45] | Phase 3, pending approval |
| ABP-938 | NCT04270747[46] | Phase 3 |
| SOK583A1 | NCT04864834[47] | Phase 3 |
| CT-P42 | NCT04739306[48] | Phase 3 |
| SCD411 | NCT04480463[49] | Phase 3 |
| AVT06 | NCT05155293[50] | Phase 3, recruiting |
| ALT-L9 | NCT04058535[51] | Phase 1, completed |
References
- ↑ Jump up to: 1.0 1.1 Chauhan MZ, Rather PA, Samarah SM, Elhusseiny AM, Sallam AB. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells. 2022 Jun 17;11(12):1950
- ↑ Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185-222. doi:10.1159/000458539
- ↑ The Eye Diseases Prevalence Research Group*. Prevalence of Age-Related Macular Degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. doi:10.1001/archopht.122.4.564
- ↑ Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi:10.1016/j.ophtha.2011.12.039
- ↑ Elhusseiny AM, Chauhan MZ, Rather PA, Samarah SM, Sallam AB. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells. 2022;11(12):1950. doi:10.3390/cells11121950
- ↑ Sharma S, Gupta V, Maiti A, et al. Safety and efficacy of Razumab™ (world's first biosimilar ranibizumab) in wet age-related macular degeneration: a post-marketing, prospective ASSET study. Int J Retina Vitreous. 2021;7(1):24. Published 2021 Mar 24. doi:10.1186/s40942-021-00293-w
- ↑ Hatamnejad A, Dadak R, Orr S, Wykoff C, Choudhry N. Systematic review of efficacy and meta-analysis of safety of ranibizumab biosimilars relative to reference ranibizumab anti-VEGF therapy for nAMD treatment. BMJ Open Ophthalmol. 2023;8(1):e001205. doi:10.1136/bmjophth-2022-001205
- ↑ Jump up to: 8.0 8.1 8.2 Kaiser PK, Schmitz-Valckenberg MS, Holz FG. ANTI–VASCULAR ENDOTHELIAL GROWTH FACTOR BIOSIMILARS IN OPHTHALMOLOGY. Retina. 2022;42(12):[[1]]. doi:10.1097/IAE.0000000000003626
- ↑ Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP. Avastin doesn't blind people, people blind people. Am J Ophthalmol. 2012;153(2):196-203.e1. doi:10.1016/j.ajo.2011.11.023
- ↑ Kumar A, Tripathy K, Chawla R. Intraocular use of bevacizumab in India: An issue resolved?. Natl Med J India. 2017;30(6):345-347. doi:10.4103/0970-258X.239079
- ↑ Jump up to: 11.0 11.1 11.2 Zhang C, Friedman S, Mruthyunjaya P, Parikh R. The Biosimilar Paradox: How Anti-VEGF Biosimilars will Increase Patient and Overall Healthcare Costs. Ophthalmology. 2023;0(0). doi:10.1016/j.ophtha.2023.04.019
- ↑ Jump up to: 12.0 12.1 Ishii-Watabe A, Kuwabara T. Biosimilarity assessment of biosimilar therapeutic monoclonal antibodies. Drug Metab Pharmacokinet. 2019;34(1):64-70. doi:10.1016/j.dmpk.2018.11.004
- ↑ Kiss S, Krawitz J, Fine HF. Coming of Age: Biosimilars. Ophthalmic Surg Lasers Imaging Retina. 2018;49(3):162-165. doi:10.3928/23258160-20180221-02
- ↑ Jump up to: 14.0 14.1 14.2 Sharma A, Reddy P, Kuppermann BD, Bandello F, Loewenstein A. Biosimilars in ophthalmology: “Is there a big change on the horizon?” Clin Ophthalmol. 2018;Volume 12:[[2]]. doi:10.2147/OPTH.S180393
- ↑ Entine J. FDA Balances Costs, Patient Safety in the Biologics and Personalized Medicine Revolution. Forbes. Accessed June 22, 2023. https://www.forbes.com/sites/jonentine/2012/07/23/fda-balances-costs-patient-safety-in-the-biologics-and-personalized-medicine-revolution-will-it-get-it-right-or-damage-the-miracle-industry/
- ↑ DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20-33. doi:10.1016/j.jhealeco.2016.01.012
- ↑ Jump up to: 17.0 17.1 Kaiser, Peter K. MD*; Schmitz-Valckenberg, Marc Steffen MD†,‡; Holz, Frank G. MD†. ANTI–VASCULAR ENDOTHELIAL GROWTH FACTOR BIOSIMILARS IN OPHTHALMOLOGY. Retina 42(12):p [[3]], December 2022. | DOI: 10.1097/IAE.0000000000003626
- ↑ McDonnell S, Principe RF, Zamprognio MS, et al. Challenges and Emerging Technologies in Biomanufacturing of Monoclonal Antibodies (mAbs). In: Biotechnology - Biosensors, Biomaterials and Tissue Engineering Annual Volume 2023. IntechOpen; 2022. doi:10.5772/intechopen.108565
- ↑ Williams GA, Repka MX, Glasser DB. The Promise and Perils of Biosimilars in Ophthalmology. Ophthalmol Retina. 2022;6(7):537-539. doi:10.1016/j.oret.2022.03.018
- ↑ Jump up to: 20.0 20.1 Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. U.S. Depertment of Health and Human Services Food and Drug Administration; 2015. https://www.fda.gov/media/82647/download
- ↑ Kim E, Han J, Chae Y, et al. Evaluation of the Structural, Physicochemical, and Biological Characteristics of SB11, as Lucentis® (Ranibizumab) Biosimilar. Ophthalmol Ther. 2022;11(2):639-652. doi:10.1007/s40123-022-00453-7
- ↑ Woo SJ, Veith M, Hamouz J, et al. Efficacy and Safety of a Proposed Ranibizumab Biosimilar Product vs a Reference Ranibizumab Product for Patients With Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2021;139(1):68. doi:10.1001/jamaophthalmol.2020.5053
- ↑ Bressler NM, Veith M, Hamouz J, et al. Biosimilar SB11 versus reference ranibizumab in neovascular age-related macular degeneration: 1-year phase III randomised clinical trial outcomes. Br J Ophthalmol. 2023;107(3):384-391. doi:10.1136/bjophthalmol-2021-319637
- ↑ Kaiser PK, Yun J, Kim S, Kim J, Park SJ. Physicochemical and Biological Stability Assessment of SB11 (Ranibizumab Biosimilar) Under Ambient and In-Use Storage for Intravitreal Administration. Ophthalmol Ther. 2023;12(2):985-998. doi:10.1007/s40123-022-00645-1
- ↑ Holz FG, Oleksy P, Ricci F, Kaiser PK, Kiefer J, Schmitz-Valckenberg S. Efficacy and Safety of Biosimilar FYB201 Compared with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2022;129(1):54-63. doi:10.1016/j.ophtha.2021.04.031
- ↑ Yoon CK, Oh J, Bae K, Park UC, Yu KS, Yu HG. Efficacy and safety of a new ranibizumab biosimilar CKD-701 using a pro re nata treatment regimen in neovascular age-related macular degeneration: A phase 3 randomized clinical trial. Vavvas DG, ed. PLOS ONE. 2022;17(11):e0275611. doi:10.1371/journal.pone.0275611
- ↑ MA KPB. Ranibizumab Biosimilar XSB-001 Has Similar Safety Profile to Reference Product. Ophthalmology Advisor. Published May 31, 2023. Accessed June 20, 2023. https://www.ophthalmologyadvisor.com/topics/retina-vitreous/xsb-001-establishes-biosimilarity-to-ranibizumab/
- ↑ Loewenstein A, Czumbel N, Ernest J, Dusová J, Pearlman J, Nowosielska A. Randomized Trial of Biosimilar XSB-001 Versus Reference Ranibizumab in Patients With Neovascular Age-Related Macular Degeneration. Ophthalmol Retina. Published online May 2023:S246865302300204X. doi:10.1016/j.oret.2023.05.005
- ↑ » XlucaneTM meets the primary endpoint in the pivotal Phase III trial with XlucaneTM – regulatory submission in EU and US planned for second half of 2021. Accessed June 20, 2023. https://xbrane.com/en/mfn_news/xlucane-meets-the-primary-endpoint-in-the-pivotal-phase-iii-trial-with-xlucane-regulatory-submission-in-eu-and-us-planned-for-second-half-of-2021/
- ↑ Xbrane Biopharma AB. A Phase III Double-Blind, Parallel Group, Multicenter Study to Compare the Efficacy and Safety of Xlucane Versus Lucentis® in Patients With Neovascular Age-Related Macular Degeneration. clinicaltrials.gov; 2022. Accessed June 19, 2023. https://clinicaltrials.gov/ct2/show/NCT03805100
- ↑ Sharma S, Khan MA, Chaturvedi A, RE-ENACT Study Investigators Group. Real-Life Clinical Effectiveness of Razumab® (the World’s First Biosimilar of Ranibizumab) in Retinal Vein Occlusion: A Subgroup Analysis of the Pooled Retrospective RE-ENACT Study. Ophthalmologica. 2019;241(1):24-31. doi:10.1159/000488602
- ↑ Advisory issued against retinal disease drug. The Times of India. https://timesofindia.indiatimes.com/business/india-business/advisory-issued-against-retinal-disease-drug/articleshow/68005152.cms. Published February 15, 2019. Accessed June 20, 2023.
- ↑ Chakraborty D, Mondal S, Boral S, et al. Biosimilar versus InnovAtor MoLecule of RAnibizumab in Neovascular Age-Related MaCular DEgeneration (The BALANCE Trial): Real-World Evidence. Clin Ophthalmol Auckl NZ. 2023;17:1067-1076. doi:10.2147/OPTH.S407219
- ↑ Singh R, Chauhan R, Saxena A, et al. A prospective, randomized, parallel group, double blind, multicenter study to compare the efficacy, safety and immunogenicity of Lupin’s Ranibizumab with Lucentis® in patients with neovascular age-related macular degeneration. Indian J Ophthalmol. 2022;70(8):[[4]]. doi:10.4103/ijo.IJO_2118_21
- ↑ Woo SJ, Bradvica M, Vajas A, et al. Efficacy and Safety of the Aflibercept Biosimilar SB15 in Neovascular Age-Related Macular Degeneration: A Phase 3 Randomized Clinical Trial. JAMA Ophthalmol. Published online June 8, 2023. doi:10.1001/jamaophthalmol.2023.2260
- ↑ Jump up to: 36.0 36.1 Nguyen NX, Olsen TA, Sheingold SH, Lew ND. MEDICARE PART B DRUGS: TRENDS IN SPENDING AND UTILIZATION, 2008-202. Off Health Policy. Published online June 9, 2023.
- ↑ Yanagi Y, Takahashi K, Iida T, et al. Cost-Effectiveness Analysis of Ranibizumab Biosimilar for Neovascular Age-Related Macular Degeneration in Japan. Ophthalmol Ther. 2023;12(4):2005-2021. doi:10.1007/s40123-023-00715-y
- ↑ Blick SKA, Keating GM, Wagstaff AJ. Ranibizumab: Drugs. 2007;67(8):1199-1206. doi:10.2165/00003495-200767080-00007
- ↑ Research C for DE and. Review and Approval. FDA. Published online December 13, 2022. Accessed June 22, 2023. https://www.fda.gov/drugs/biosimilars/review-and-approval
- ↑ Biogen and Samsung Bioepis’ BYOOVIZTM (ranibizumab-nuna) Launches in the United States | Biogen. Accessed June 22, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-and-samsung-bioepis-byooviztm-ranibizumab-nuna-launches
- ↑ Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2015;122(10):2044-2052. doi:10.1016/j.ophtha.2015.06.017
- ↑ Research C for DE and. Review and Approval. FDA. Published online December 13, 2022. Accessed June 22, 2023. https://www.fda.gov/drugs/biosimilars/review-and-approval
- ↑ A Study to Compare SB15 (Proposed Aflibercept Biosimilar) to Eylea in Subjects With Neovascular Age-related Macular Degeneration (AMD) - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04450329
- ↑ Comparative Study to Evaluate the Efficacy and Safety of MYL-1701P and Eylea® in Subjects With Diabetic Macular Edema (DME) - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT03610646
- ↑ Comparative Study to Evaluate the Efficacy and Safety of MYL-1701P and Eylea® in Subjects With Diabetic Macular Edema (DME) - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT03610646
- ↑ A Study to Understand Effectiveness and Safety of ABP 938 Compared to Aflibercept (Eylea®) in Patients Suffering With Neovascular Age-related Macular Degeneration [Neovascular (Wet) AMD] - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04270747
- ↑ Phase III Study Assessing the Efficacy, Safety and Immunogenicity of SOK583A1 Versus Eylea® in Patients With Neovascular Age-related Macular Degeneration - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04864834
- ↑ Study to Compare Efficacy and Safety of CT-P42 in Comparison With Eylea in Patients With Diabetic Macular Edema - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04739306
- ↑ A Study to Comparing SCD411 and Eylea® in Subjects With Wet Age-related Macular Degeneration (AMD) - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04480463
- ↑ Clinical Study to Compare Efficacy and Safety of AVT06 and EU-Eylea (ALVOEYE) - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT05155293
- ↑ Clinical Study of ALT-L9 to Determine Safety, Efficacy and Pharmacokinetics in Neovascular AMD - Full Text View - ClinicalTrials.gov. Accessed June 22, 2023. https://clinicaltrials.gov/ct2/show/NCT04058535

