Avacincaptad
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Avacincaptad pegol intravitreal solution (Izervay; Astellas Pharma Inc) was approved in 2023 in the United States as a treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Astellas Pharma announced withdrawing its marketing authorization application for avacincaptad pegol from the European Medicines Agency (EMA) in October 2024.
Background
Dry age-related macular degeneration can develop into an advanced stage called geographic atrophy, which causes irreversible loss of vision.[1] GA affects approximately 5 million people globally, and its rate of incidence increases with age.[2] Patients with this condition typically struggle with basic visual functions such as reading and dim-light vision due to loss of retinal pigment epithelium (RPE), photoreceptors and choriocapillaris in the macula.[1][3][4] In 2023, Pegcetacoplan (Syfovre) emerged as the first treatment for GA quickly followed by a second treatment, avacincaptad pegol intravitreal solution (Izervay), giving patients another option to potentially slow vision loss.[5]
Disease Entity
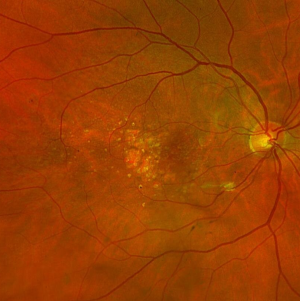
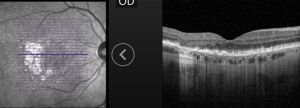
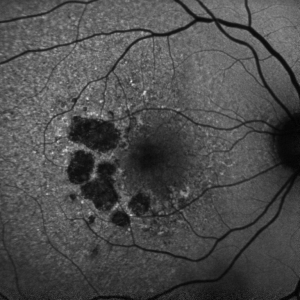
GA is a complex pathophysiologic process that is defined by sharply demarcated atrophic lesions of the outer retina, resulting from the loss of photoreceptors, RPE, and choriocapillaris in the macula.[1] . On fundus evaluation (Figure 1), scalloped areas of hypopigmentation will be found in areas of atrophy with surrounding drusen and pigmentary changes. Fundus autofluorescence (Figure 2) reveals dense hypoautofluorescence due to the lack of lipofuscin following loss of the outer retina and RPE. The amount of perilesional hyperautofluorescence can be used to predict disease progression, with more hyperautofluorescence generally being proportional to the rate of GA growth. Certain patterns of hyperautofluorescence have been implicated in faster disease progression. Optical coherence tomography demonstrates loss of outer retinal layers (outer nuclear layer, ellipsoid zone, interdigitation zone), as well as loss of the underlying RPE and choroidal hypertransmission (Figure 3). In 2017, the Classification of Atrophy Meetings group recommended that non-neovascular AMD trials include color fundus photographs, fundus autofluroescence, near-infrared reflectance, and spectral-domain or swept-source OCT[6], all of which are good tools to help diagnose and monitor disease progression.
Mechanism of Action
Avacincaptad targets complement factor C5 within the complement system. The complement cascade plays a key role in the innate immune system and is initiated through the classic (antibody-dependent), the alternative (antibody-independent), and the lectin pathways. These activation pathways result in activation of the same end-product, C3 convertase, which cleaves complement C3 leading to the generation of complement C5 and cleavage of C5 into C5a and C5b. Ultimately, C5a and C5b play key roles in cell death[7][8].
Complement factor C5 is involved in inflammatory processes in AMD based on genetic studies, preclinical studies, clinical studies and immunohistochemical studies[7][9][10][11]. Plasma concentrations of C3a, C5a and C5 were elevated in AMD patients compared to control patients[9]. By inhibiting complement C5 formation, inflammation leading to GA is hypothesized to slow down.
Avacincaptad pegol is a pegylated RNA aptamer, which functions as a chemical antibody against complement C5, and inhibits the cleavage of complement C5 into its fragments (C5a and C5b). As such, the involvement of Avacincaptad pegol in the inhibition of the complement cascade slows down the progression of GA secondary to AMD[7].
Clinical Trials
The GATHER 1 phase 2/3 clinical trial with 286 patients and GATHER2 phase 3 clinical trial with 447 patients investigated the efficacy and safety of Avacincaptad pegol. In GATHER1, the patients were randomized in two parts. The first part the patients randomized to receive 1mg, 2 mg or sham every 4 weeks. In the second part the patients randomized again and given 2mg, 4mg or a sham every 4 weeks[12]. In GATHER2, the patients were either given a sham or 2 mg intravitreal injection of Avacincaptad pegol every 4 weeks[4].
Clinical Efficacy
GATHER1 and GATHER2 results showed a reduced growth in GA compared their respective sham group[13][14][15] (Table 1).
Table 1. GA Lesion Growth in GATHER1 and GATHER2[13][14][15].
| Primary Efficacy Endpoint analysis | GATHER1 | GATHER2 | ||
|---|---|---|---|---|
| Izervay | Sham | Izervay | Sham | |
| Percent in GA Growth | 1.22 | 1.89 | 1.75 | 2.12 |
| Difference (95% CI) (mm2/year)
Difference P Value |
0.67 (0.21 -1.13)
35% <0.01 |
0.38 (0.12-0.63)
18% <0.01 | ||
GATHER2 results showed that when 2mg intravitreal Avacincaptad pegol and sham were given over the course of 12 months, the GA rate of growth reduced 14.3% more in the Avacincaptad pegol group compared to the sham group using square root transformation analysis[12].
During post-hoc analysis of GATHER2 comparing geographic regional differences between the US and the rest of the world, researchers noted that U.S. participants had a mean growth reduction of 25.5% and a baseline lesion that was 13% smaller compared to the rest of the world. A possible reason for this difference is the earlier stage of disease treated in the US during this trial, with possibly a greater benefit earlier in the course of disease[13][14][15].
Contraindications and Adverse Events
Avacincaptad pegol should not be used in patients with ocular or periocular infections, nor active intraocular inflammation. Common adverse effects include but are not limited to conjunctival hemorrhage, increased IOP, blurred vision, choroidal neovascularization, eye pain, vitreous floaters and blepharitis[14][15]. This is summarized in table 2.
Table 2. Side Effects seen in GATHER1 and GATHER2 combined[14][15].
| Adverse Drug Reactions | Izervay | Sham |
|---|---|---|
| Conjunctival hemorrhage | 13% | 9% |
| Increased IOP | 9% | 1% |
| Blurred Vision | 8% | 5% |
| Choroidal neovascularization | 7% | 4% |
| Eye Pain | 4% | 3% |
| Vitreous Floaters | 2% | <1% |
| Blepharitis | 2% | <1% |
The most frequently reported ocular adverse events were related to the injection procedure. There were no intraocular inflammation or ischemic optic neuropathy events over 12 months[13][16]. The emergence of vasculitis cases after the launch of the first GA medication, pegcetacoplan, highlights the unknown risks that can develop with any new treatment.
Researchers saw increased rates of neovascular AMD (choroidal neovascularization) in clinical trials[1]. The patients that developed macular neovascularization (MNV) during the trial were dropped from the study, so the details of the drug’s effect on clinical course and impact on BCVA are limited. MNV conversion rates were 11.9% with ACP 2 mg and 15.7% with ACP 4 mg, compared to 2.7% and 2.4% in their respective sham groups in the GATHER1 trial. The predominant MNV noted by researchers was type 2 macular neovascularization. Fellow-eye conversion rates were 3.0%, 3.6%, and 3.6% for ACP 2 mg, 4 mg, and sham, respectively. Investigators preemptively decided when creating the trials to dismiss participants who developed MNV as they would prevent accurate FAF measurements. The mechanism behind the development of MNV is not known. Some hypothetical mechanisms include increased levels of VEGF-A producing cells, proliferation of pro-angiogenic M2 macrophages, and reduced choriocapillaris apoptosis through complement inhibition[17].
Injection: Dosing, Administration, Preparation
Avacincaptad pegol is packaged as a 20mg/mL solution in a single-dose vial stored at temperatures between 2 ºC to 8ºC[14][15]. The recommended dosing for the medication is 2 mg or 0.1mL of the 20 mg/mL solution. The contents of the vial are pulled up through a large-bore needle without a filter. The loading of the medication into a 1 CC syringe is like other medications. The medication is typically injected through a 30-gauge needle. Before injection, the eye should be anesthetized and prepped in usual intravitreal injection fashion.
More information on injection preparation and administration can be found on the FDA label: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217225s000lbl.pdf
Patient Selection
As with any treatment in medicine, the provider must navigate patients through the risks, benefits, and treatment burden of therapy. Due to the relatively mild reduction in GA growth and safety considerations, some patients and providers are understandably hesitant to pursue aggressive treatment with Avacincaptad[14][15][18]. Others are excited by the possibility to slow down a previously untreatable condition. Numerous factors impact clinical decision making to pursue treatment, including the patient’s baseline visual function, cognitive function, goals of care, life expectancy, social support, risk aversion, injection tolerability and general sense towards treatment. With time, providers will develop a better understanding of which patients are best suited for this novel treatment.
Conclusions
Avacincaptad pegol is an FDA approved medication for GA secondary to AMD, a previously untreatable condition. Over two phase 3 clinical trials, it has been proved to slow GA lesion growth rate by targeting complement factor C5. The reduction in growth rate is limited, so providers will need to help navigate patients through the risks, benefits, expectations and treatment burden when deciding on pursuing treatment.
References
- ↑ 1.0 1.1 1.2 1.3 Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, Henry EC, Ferrara D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018 Mar;125(3):369-390. doi: 10.1016/j.ophtha.2017.08.038. Epub 2017 Oct 27. PMID: 29110945.
- ↑ Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014 Feb;2(2):e106-16. doi: 10.1016/S2214-109X(13)70145-1. Epub 2014 Jan 3. PMID: 25104651.
- ↑ Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, Haselwood D. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997 Oct;104(10):1677-91. doi: 10.1016/s0161-6420(97)30079-7. PMID: 9331210; PMCID: PMC2730508.
- ↑ 4.0 4.1 Khan H, Aziz AA, Sulahria H, Khan H, Ahmed A, Choudhry N, Narayanan R, Danzig C, Khanani AM. Emerging Treatment Options for Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. Clin Ophthalmol. 2023 Jan 23;17:321-327. doi: 10.2147/OPTH.S367089. PMID: 36741078; PMCID: PMC9892637.
- ↑ Apellis Pharmaceuticals. SyfovreTM (Pegcetacoplan Injection). Syfovre. 2023. https://syfovre.com/.
- ↑ Holz FG, Sadda SR, Staurenghi G, Lindner M, Bird AC, Blodi BA, Bottoni F, Chakravarthy U, Chew EY, Csaky K, Curcio CA, Danis R, Fleckenstein M, Freund KB, Grunwald J, Guymer R, Hoyng CB, Jaffe GJ, Liakopoulos S, Monés JM, Oishi A, Pauleikhoff D, Rosenfeld PJ, Sarraf D, Spaide RF, Tadayoni R, Tufail A, Wolf S, Schmitz-Valckenberg S; CAM group. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology. 2017 Apr;124(4):464-478. doi: 10.1016/j.ophtha.2016.12.002. Epub 2017 Jan 18. PMID: 28109563.
- ↑ 7.0 7.1 7.2 Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, Joondeph BC, Randolph J, Masonson H, Rezaei KA. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021 Apr;128(4):576-586. doi: 10.1016/j.ophtha.2020.08.027. Epub 2020 Sep 1. PMID: 32882310
- ↑ Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32:433-59. doi: 10.1146/annurev-immunol-032713-120154. Epub 2014 Jan 29. PMID: 24499275.
- ↑ 9.0 9.1 Scholl HP, Charbel IP, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3(7):e2593. doi:10.1371/journal.pone.0002593
- ↑ Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005 Apr 15;308(5720):385-9. doi: 10.1126/science.1109557. Epub 2005 Mar 10. PMID: 15761122; PMCID: PMC1512523.
- ↑ Cao J, Zhang F, Xiong W. Discovery of Aptamers and the Acceleration of the Development of Targeting Research in Ophthalmology. Int J Nanomedicine. 2023 Aug 2;18:4421-4430. doi: 10.2147/IJN.S418115. PMID: 37551274; PMCID: PMC10404440.
- ↑ 12.0 12.1 “Iveric Bio Announces Positive Zimura 18 Month Data Supporting the 12 Month Efficacy Findings: Continuous Positive Treatment Effect with Favorable Safety Profile in Geographic Atrophy Secondary to Age-Related Macular Degeneration in a Phase 3 Trial.” Iveric Bio, investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-zimura-18-month-data-supporting-12. Accessed 10 Sept. 2023.
- ↑ 13.0 13.1 13.2 13.3 “Iveric Bio Announces Positive Topline Data from Zimura® GATHER2 Phase 3 Clinical Trial in Geographic Atrophy.” Iveric Bio, 9 June 2022, investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-topline-data-zimurar-gather2-phase.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Izervay FDA Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217225s000lbl.pdf
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Highlights of prescribing information - Iveric. August 2023. https://ivericbio.com/wp-content/uploads/IZERVAY-avacincaptad-pegol-intravitreal-solution-PI_Final_8.4.23.pdf.
- ↑ Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Khanani, Arshad MAlezzandrini, Arturo et al. The Lancet, Volume 0, Issue 0
- ↑ Patel, S.S., Lally, D.R., Hsu, J. et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye (2023). https://doi.org/10.1038/s41433-023-02497-w
- ↑ Cruz-Pimentel M, Wu L. Complement Inhibitors for Advanced Dry Age-Related Macular Degeneration (Geographic Atrophy): Some Light at the End of the Tunnel? J Clin Med. 2023 Aug 4;12(15):5131. doi: 10.3390/jcm12155131. PMID: 37568533; PMCID: PMC10420150.