Autosomal Dominant Optic Atrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Autosomal dominant optic atrophy (ADOA) is estimated to be the most common hereditary optic neuropathy with an estimated disease prevalence of 1:12,000 to 1:50,000 [1].
Disease
The typical onset of visual loss is in the first or second decade of life, although most patients cannot identify a precise onset of reduced acuity due to the gradual progression [2]. Visual acuity loss is commonly bilateral and relatively symmetric with a mild, slow, and insidious course. More than 80 % of patients maintain better than 20/200 vision, although there is wide interfamilial and intrafamilial variation with incomplete penetrance [2][3][4]. Color vision deficits, such as tritanopia, are frequently present [5]. Some Optic Atrophy 1 (OPA1) mutation carriers present a DOA plus phenotype, which includes hearing loss, peripheral neuropathy, myopathy, ataxia, and chronic progressive external ophthalmoplegia [6][7][8].
Pathophysiology
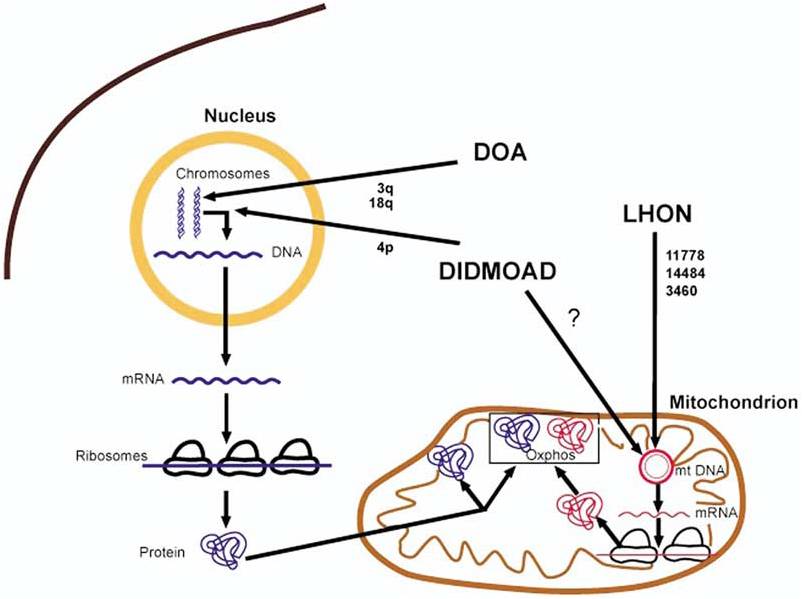
ADOA is commonly associated with mutations in the nuclear OPA1 gene located on chromosome 3q28-q29. OPA1 encodes for a mitochondrial dynamin-related GTPase that is involved in mitochondrial membrane biogenesis and stabilization of membrane integrity [2][5][9]. Abnormal mitochondrial metabolism and impaired oxidative phosphorylation lead to increases in reactive oxygen species levels and retinal ganglion cell apoptosis [6][10]. Retinal ganglion degeneration, predominantly in the papillomacular bundle, has been suggested as an underlying defect [9].
Figure 1 [2]. Hereditary optic neuropathies and proposed common pathophysiology through mitochondrial dysfunction. The bold arrows indicated where the genetic defects underlying dominant optic atrophy (DOA), Leber hereditary optic neuropathy (LHON), and Wolfram syndrome (DIDMOAD) may cause mitochondrial dysfunction and optic atrophy. mRNA=messenger RNA; mtDNA=mitochondrial DNA; oxphos=oxidative phosphorylation.
Diagnosis
Individuals typically present with bilateral and slow vision loss starting in the first or second decade of life. Family history of similar presentation is common, but may be absent due to considerable interfamilial and intrafamilial variation in visual acuity. Thus, optic atrophy with little or no vision loss is frequently discovered in otherwise undiagnosed and “asymptomatic” family members.
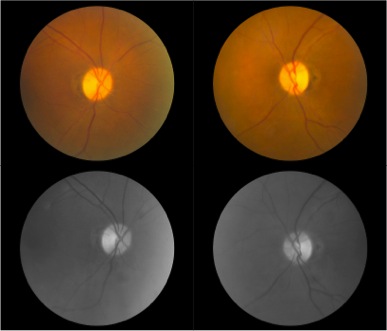
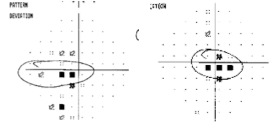
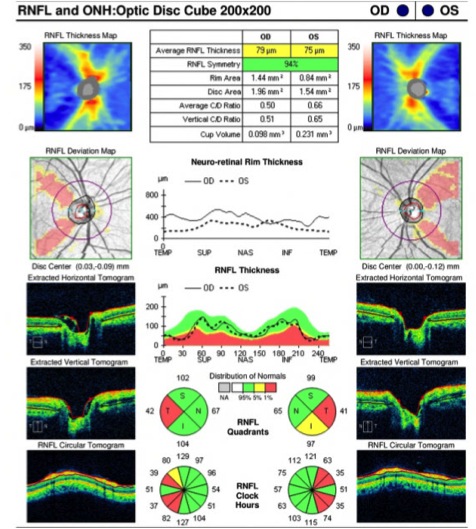
Optic disc atrophy typically shows focal, wedged-shaped temporal optic atrophy, however diffuse atrophy may be present. As the primary pathology is the papillomacular bundle, central, centrocecal and paracentral scotomas are the most common visual field defects. Color vision deficits are common. Visual-evoked responses in affected individuals show diminished amplitudes and prolonged latencies. Pattern electroretinograms show a reduced N95 component [5].
Figure 2. Both optic nerve heads present temporal pallor as seen on fundus photography and are often more prominent at red-free fundus photo. Humphrey visual field test shows cecocentral scotoma in the right eye and central scotoma in the left eye. Optic coherence tomography (OCT) evaluating retinal nerve fiber layer (RNFL) shows decreased average thickness predominantly at temporal aspect in both eyes. Optic coherence tomography angiography (OCTA) may reveal macular and peripapillary neurovascular changes in patients [11].
Laboratory test
Although OPA 1 is known to be the major gene involved, because of the large number of different causative mutations, a simple, rapid DNA test, such as that used for LHON, may not be possible except in the families with known mutations [2].
Differential Diagnosis [5][9]
• Leber’s Hereditary Optic Neuropathy (LHON)
• Nutritional Amblyopia
• Toxic Optic Neuropathy
• Demyelinative disease
• Ocular dysthyroidism
• Macular Dystrophies
• Glaucoma
Management
There is no established medical treatment for ADOA. ADOA is regarded as one of the two classic paradigms of mitochondrial dysfunction in optic neuropathies (LHON being the other). Coenzyme Q-10 (CoQ), Idebenone, and nutritional supplements, such as vitamin B12 and C and lutein, have been suggested to reduce reactive oxygen species induced stress in the optic nerve [12]. Topical agents deemed to be neuroprotective or antiapoptotic, such as brimonidine, have also been recommended although, evidence of their efficacy remains anecdotal [12]. Annual assessment of visual acuity, visual fields, color vision, extraocular muscles, and hearing evaluation is recommended after the diagnosis of ADOA [13].
References
- ↑ Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. II. Clinical and epidemiological aspects. Acta Ophthalmol Scand. 1996;74(1):3-7.
- ↑ 2.0 2.1 2.2 2.3 2.4 Newman NJ. Hereditary Optic Neuropathies: from the mitochondria to the optic nerve. Am J Ophthal. 2005;140(3):517-523.
- ↑ Votruba M, Fitzke FW, Holder CE, Carter A, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol. 1998;116(3):353-358.
- ↑ Cohn AC, Toomes C, Potter C, Towns KV, Hewitt AW, Inglehearn CF et al. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007; 143(4): 656–662.e651.
- ↑ 5.0 5.1 5.2 5.3 Kline LB, Glaser JS. Dominant optic atrophy: The clinical profile. Arch Ophthalmol. 1979;97(9):245-251.
- ↑ 6.0 6.1 Chun BY, Rizzo JF 3rd. Dominant Optic Atrophy and Leber's Hereditary Optic Neuropathy: Update on Clinical Features and Current Therapeutic Approaches. Semin Pediatr Neurol. 2017 May;24(2):129-134.
- ↑ Yu-Wai-Man P, Votruba M, Moore AT, Chinnery PF. Treatment strategies for inherited optic neuropathies: past, present and future. Eye (Lond). 2014 May;28(5):521-37.
- ↑ Skidd PM, Lessell S, Cestari DM. Autosomal dominant hereditary optic neuropathy (ADOA): a review of the genetics and clinical manifestations of ADOA and ADOA+. Semin Ophthalmol. 2013 Sep-Nov;28(5-6):422-6.
- ↑ 9.0 9.1 9.2 Delettre C, Lenaers G, Pelloquin L, Belenguer P, Hamel CP. OPA1 (Kjer type) dominant optic atrophy: a novel mitochondrial disease. Mol Genet Metab. 2002;75(2):97-107.
- ↑ Chun BY, Rizzo JF 3rd. Dominant optic atrophy: updates on the pathophysiology and clinical manifestations of the optic atrophy 1 mutation. Curr Opin Ophthalmol. 2016 Nov;27(6):475-480.
- ↑ Martins A, Rodrigues TM, Soares M, Dolan MJ, Murta JN, Silva R, Marques JP. Peripapillary and macular morpho-vascular changes in patients with genetic or clinical diagnosis of autosomal dominant optic atrophy: a case-control study. Graefes Arch Clin Exp Ophthalmol. 2019 May;257(5):1019-1027.
- ↑ 12.0 12.1 Carelli V, La Morgia C, Sadun AA. Mitochondrial dysfunction in optic neuropathies: animal models and therapeutic options. Curr Opin Neurol. 2013;26(1):52-58.
- ↑ Ham M, Han J, Osann K, Smith M, Kimonis V. Meta-analysis of genotype-phenotype analysis of OPA1 mutations in autosomal dominant optic atrophy. Mitochondrion. 2019 May;46:262-269.