Aponeurotic Ptosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Aponeurotic Ptosis [a-pə-nu-ˈrä-tik ˈtō-səs] is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
- ICD-9
- 374.3 Ptosis of eyelid
- 374.30 Ptosis of eyelid, unspecified
- ICD-10
- H02.4 Ptosis of eyelid
- H02.401 Acquired ptosis of right eyelid
- H02.402 Acquired ptosis of left eyelid
- H02.403 Acquired ptosis of bilateral eyelids
- H02.4 Ptosis of eyelid
Disease
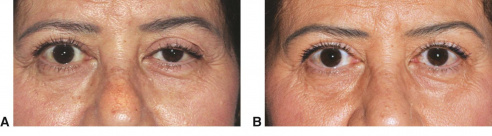
Ptosis (or Blepharoptosis) is the drooping of the upper eyelid margin. It is a common cause of reversible peripheral vision loss that affects the superior visual field first and then can go on to affect central vision. Patients may also report difficulty with reading, as certain types of ptosis can worsen when eyes are in downgaze. Patients can develop ptosis from birth (congenital) or later during life (acquired). Ptosis can also be classified by etiology: myogenic, neurogenic, mechanical, traumatic, or aponeurotic. The last is the subject of this article.
Aponeurotic ptosis is the most common type of acquired ptosis and the most common cause of ptosis overall. It is also known as senile or involutional ptosis, because it occurs most often in the elderly as an involutional disorder, meaning related to aging. This entity was first described by Jones, Quickert, and Wobig in 1975, who demonstrated that the levator aponeurosis appeared dehisced or disinserted from its normal position on the tarsus[1]
Etiology
The levator aponeurosis is a fascial tissue that connects the levator palpebrae superioris muscle (levator muscle) to the tarsus, a thick plate of connective tissue that lies in the upper eyelid, as well as to the overlying skin. The levator aponeurosis transmits the force of the levator muscle to lift the upper eyelid. Any dehiscence, disinsertion, or stretching of the levator aponeurosis, either congenital or acquired, can lead to ptosis.
Common causes are involutional attenuation or repetitive traction on the eyelid. The latter is commonly seen with those that rub their eyelids frequently or in cases of contact lens use. Aponeurotic ptosis may be further worsened by eye surgery or procedures.
Congenital aponeurotic ptosis is uncommon. Most cases of aponeurotic ptosis occurring from birth are secondary to trauma during delivery.
Risk Factors
Risk factors for aponeurotic ptosis occurring from birth include forceps delivery, vacuum extraction, traumatic fetal rotation, and shoulder dystocia. Risk factors for aponeurotic ptosis occurring later in life include chronic contact lens use, inflammatory diseases, trauma, intraocular surgery, or frequent eye rubbing, as commonly seen in atopic individuals and in those with Down’s syndrome. The incidence of ptosis following cataract surgery was found to be 7.3% in one study[2].
General Pathology
The primary changes found in acquired aponeurotic ptosis include dehiscence or disinsertion of the levator aponeurosis from the tarsus and dehiscence of the medial limb of Whitnall’s ligament from connective tissue at the medial orbital rim[3].
A study that used ultrasound biomicroscopy to measure the thickness of the levator aponeurosis confirmed that the levator aponeurosis thickness in eyelids with aponeurotic ptosis is much thinner than that of the normal eyelid[4].
Histopathology
Histopathological slides from the eyelids of patients with aponeurotic ptosis were evaluated, revealing that 71% of aponeuroses showed disinsertion and 12% showed attenuation of the aponeurosis (the remainder showed inconclusive changes). The remaining 17% were inconclusive. Müller’s muscle remained largely unchanged in these patients[5].
Diagnosis
Symptoms
Patients with aponeurotic ptosis may present with a spectrum of symptoms, ranging from visually asymptomatic cosmetic eyelid asymmetry to visually significant obstruction. While the superior visual field is most commonly obstructed, central vision can also be obstructed. In addition, patients may also report trouble with reading, as aponeurotic ptosis worsens in downgaze. Patients tend to compensate with overaction of the frontalis muscle, however, persistent brow elevation may lead to frontalis fatigue and even cephalgia.
If patients report a fluctuation in symptoms or eye muscle fatigue throughout the day, statin use and ocular myasthenia gravis should be ruled out[6].
Physical examination
The physical examination of a patient with ptosis is aimed at determining etiology, eyelid muscle function through eyelid measurements, and assessment of surrounding facial structures.
Margin reflex distance 1 (MRD1): The distance from the upper eyelid margin to the corneal light reflex. This measurement is taken in primary position, with the patient fixating on the light source. Typically, MRD1 is 4-5 mm. In severe ptosis, the light reflex can be obstructed by the eyelid and the MRD1 is then zero or a negative value. In all cases, the more ptotic eyelid should be lifted to unmask occult contralateral ptosis (due to Hering’s law of equal innervation).
Margin reflex distance 2 (MRD2): The distance from the corneal light reflex to the lower eyelid margin with the patient fixating on the light source. A typical MRD2 is 4-5 mm. An increased MRD2 indicates increased eye exposure and, thus, an increased risk of post-operative dry eye symptoms.
Margin to crease distance (MCD): The distance from the upper eyelid crease to the upper eyelid margin with the patient looking down at a 45 degree angle. In Caucasians, MCD is typically 8-9 mm in males and 9-11 mm in females, while this often decreases to 2-5mm in East Asians. Aponeurotic defects characteristically have a high or an absent upper eyelid crease.
Levator function (upper eyelid excursion): The distance from the upper eyelid margin in downgaze to upgaze with frontalis muscle function neutralized. Typically, levator function is 12-17 mm.
The levator function is classified as
- Good 8 mm or greater
- Fair 5 -7 mm
- Poor less than or equal to 4 mm
Hering’s law of equal innervation: The levator muscles obey Hering’s law of equal innervation, meaning they are innervated symmetrically. In cases of asymmetric ptosis, the levator muscles will receive an equal amount of increased central neural output to compensate for the ptosis. Therefore, the less ptotic eyelid may appear to have a normal height. However, when the more ptotic eyelid is manually elevated, the decreasing neural output to both eyelids results in descent of the contralateral eyelid. An immediate fall of the contralateral eyelid confirms the presence of bilateral, asymmetrical ptosis masked by levator “overaction.” A subclinical ptosis can thus be detected and explained to patients prior to surgery. This prevents post-operative “surprises”; if the patient decides not to do surgery on the less ptotic eye, the patient then is forewarned that the non-operated eye will appear to "develop" ptosis post-operatively.
Lagophthalmos: Lagophthalmos is the inability of the upper and lower eyelids to close completely, which leaves conjunctiva and, sometimes, a portion of the cornea unprotected. If present, the gap between the eyelids should be measured and the amount of corneal exposure documented (both in millimeters). Lagophthalmos and pre-existing signs/symptoms of dry eyes may predispose patients to postoperative exposure keratopathy.
Visual acuity and refraction: Visual acuity is always important to check prior to surgery, understanding that a patient’s refraction may change post-ptosis surgery. This can occur due to corneal contour changes secondary to eyelid pressure. Corneal topography can demonstrate an increase in against-the-rule astigmatism. These changes tend to be temporary, with a return of refractive shift towards normal by 12 months after surgery8. Ophthalmologists should avoid prescribing glasses to patients prior to and up to 3 months following ptosis surgery.
Other Physical Exam Findings:
If there are accompanying pupil or extraocular movement disorders present, entities, such as Horner's syndrome, PCOM aneurysm, and other orbital disorders should be considered. Systemic disorders causing ptosis, such as Myasthenia gravis, oculopharyngeal dystrophy, and neurological disorders, should be identified in all patients prior to surgery.
- Myasthenia gravis is notable for ptosis that fatigues, worsening at the end of the day. Levator fatigability can be assessed by asking the patient to look in extreme upgaze for up to one to two minutes and to check for improvement with the rest or ice test.
- Oculopharyngeal dystrophy, a late-onset genetic myopathy is seen as slowly progressive ptosis, dysphagia, and dysphonia (difficulty with speaking), starting around age 50.
- Oculomotor (CN 3) palsy is seen in patients with ptosis, mydriasis, and an eye positioned down and outward, causing diplopia.
- Horner Syndrome, which manifests as ptosis, miosis, and anhidrosis, results from a lesion to the sympathetic pathways.
Elderly patients, who have dermatochalasis, must be assessed carefully as the redundant upper eyelid skin may appear to cause a ptosis (pseudoptosis).
Diagnostic procedures
Visual field testing with the eyelids untaped (in the natural, ptotic state) and taped (artificially elevated) can provide objective data of the patient's level of functional visual impairment. The improvement with taped eyelids estimates the visual improvement that can be expected after surgery. This can be required by insurance companies in order to ensure coverage of treatment.
External full-face photography documents the presence and progression of the ptosis.
Pharmacologic testing is used by some to determine management of ptosis. In patients with a good levator function, some surgeons use response to topical phenylephrine testing to direct surgical management, whether by anterior levator advancement versus Müller's Muscle-Conjunctival Resection.
Differential diagnosis
- Congenital Ptosis:
- Myogenic ptosis
- Idiopathic or genetic
- Blepharophimosis syndrome
- Neurogenic ptosis
- Congenital cranial nerve III palsy
- Congenital Horner syndrome
- Marcus Gunn jaw-winking syndrome
- Mechanical ptosis
- Traumatic ptosis
- Pseudoptosis
- Myogenic ptosis
- Acquired Ptosis:
- Myogenic ptosis
- Myasthenia gravis
- Chronic progressive external ophthalmoplegia
- Oculopharyngeal dystrophy
- Medication-related
- Neurogenic ptosis
- Cranial nerve III palsy
- Horner syndrome
- Mechanical ptosis
- Traumatic ptosis
- Pseudoptosis
- Myogenic ptosis
Management
Surgical Options
The mainstay of ptosis management relies on surgical correction, however the patient’s ocular, medical, and surgical history will determine if surgical repair is appropriate. The most important factor in surgical decision making is the levator muscle function.
When levator function is normal (greater than 10), this indicates that the levator muscle itself is strong and functioning normally. Tightening the levator muscle will elevate the eyelid margin.
- External (transcutaneous) levator advancement: Through an upper eyelid crease incision, the levator aponeurosis is surgically dissected from the tarsus and superiorly from the overlying orbital fat. A partial thickness suture is passed through the tarsus and through the levator muscle, resulting in an advancement of the levator muscle. This technique requires the patient’s cooperation to assess and adjust eyelid height after the surgical advancement.
- Internal (transconjunctival) levator/tarsus/Müller muscle resection approaches: Patients that demonstrate improvement in ptosis after instillation of topical phenylephrine may be candidates for the internal approach. These procedures focus on the removal of a part of the Müller muscle, the tarsus, or the levator aponeurosis to shorten the distance between the levator muscle and the tarsus, thus increasing its ability to elevate the upper eyelid. One advantage of this approach is the preservation of the external eyelid and lack of visible scar. The Müller muscle-conjunctival resection (MMCR) and the Fasanella-Servat ptosis repair procedures are examples of the internal approach.
When levator function is poor (less than 5mm), the levator muscle is not strong enough to lift the eyelid, no matter how it is manipulated, so the frontalis muscle is recruited.
- Frontalis muscle suspension: Frontalis suspension surgery, commonly called a frontalis sling, suspends the eyelid directly from the frontalis muscle, allowing the patient to elevate the eyelid by recruiting the frontalis to lift the brow and eyelid. The material used for the sling may be autogenous (harvested from the patient’s tensor fascia lata), allogenic (from banked fascia lata), or synthetic (silicone or synthetic sutures).
- Frontalis muscle advancement: In this surgery, the frontalis muscle is connected inferiorly to the tarsus, allowing the brow to directly elevate the eyelid.
Complications
Undercorrection is the most common complication of ptosis repair, which is seen in 10–15% of cases. As some component of post-operative undercorrection may be mechanical secondary to post-op eyelid edema, these patients should be observed until edema has resolved and the eyelid position has stabilized.
Other complications include overcorrection, unsatisfactory eyelid contour, surgical wound scarring or dehiscence, eyelid crease asymmetry, conjunctival prolapse, tarsal eversion, implant extrusion, infection, exposure keratopathy, and lagophthalmos. Cases of overcorrection should be observed until post-operative changes stabilize. Daily digital massage for several months can lower the eyelid, improving mild overcorrection.
Surgical revision is considered in patients with symptomatic over or undercorrection. Achieving symmetry between both eyelids is the most difficult aspect of ptosis repair and some surgeons use adjustable sutures and post-operative, in-office adjustments to attempt to achieve this goal.
Changes in corneal astigmatism can be seen in up to 72% of patients undergoing ptosis repair. It is generally with-the-rule and in most cases regresses back toward the pre-operative level within 1 year[7].
Prognosis
The majority of ptosis procedures are successful, resulting in increased upper eyelid margin positioning.
Primary prevention
Primary prevention of acquired aponeurotic ptosis focuses on the prevention of excessive tractional forces on the eyelid, such as excessive rubbing of the eye. Patients with recurrent/seasonal allergic conjunctivitis should be advised to avoid excessive eye rubbing.
The prevention of postsurgical aponeurotic ptosis is aimed at efficient surgical time. This limits trauma to the eyelid caused by ocular inflammation and use of a lid speculum[8].
References
- ↑ Jones LT, Quickert MH, Wobig JL. The Cure of Ptosis by Aponeurotic Repair. Archives of Ophthalmology. 1975;93(8):629-634. doi:10.1001/archopht.1975.01010020601008.
- ↑ Hosal B, Tekeli O, Gürsel E. Eyelid Malpositions after Cataract Surgery. European Journal of Ophthalmology. 1998;8(1):12-15. doi:10.1177/112067219800800104
- ↑ Korn, BS. 2019-2020 Basic and Clinical Science Course, Section 07: Oculofacial Plastic and Orbital Surgery eBook. https://elibrary.aao.org/epubreader/20192020-basic-clinical-science-course-section-07-oculofacial-plastic-orbital-surgery-ebook
- ↑ Hoşal BM, Ayer NG, Zilelioğlu G, Elhan AH. Ultrasound Biomicroscopy of the Levator Aponeurosis in Congenital and Aponeurotic Blepharoptosis. Ophthalmic Plastic & Reconstructive Surgery. 2004;20(4):308-311. doi:10.1097/01.iop.0000129532.33913.e7.
- ↑ Dortzbach RK. Involutional Blepharoptosis. Archives of Ophthalmology. 1980;98(11):2045. doi:10.1001/archopht.1980.01020040897022.
- ↑ Gale J, Danesh-Meyer HV. Statins can induce myasthenia gravis. Journal of Clinical Neuroscience. 2014;21(2):195-197. doi:10.1016/j.jocn.2013.11.009.
- ↑ Hoick DEE, Dutton JJ, Wehrly SR. Changes in Astigmatism After Ptosis Surgery Measured by Corneal Topography. Ophthalmic Plastic & Reconstructive Surgery. 1998;14(3):151-158. doi:10.1097/00002341-199805000-00001.
- ↑ Crum AV. Preventing & Managing Post-Surgical Ptosis. Review of Ophthalmology. https://www.reviewofophthalmology.com/article/preventing-managing-post-surgical-ptosis. Published October 9, 2010. Accessed April 19, 2020.
- Ahmad K, Wright M, Lueck CJ. Ptosis. Practical Neurology. 2011;11(6):332-340. doi:10.1136/practneurol-2011-000026.
- Boughton B. Assessing and Correcting Ptosis. American Academy of Ophthalmology. https://www.aao.org/eyenet/article/assessing-correcting-ptosis?novemberdecember-2007. Published March 23, 2016. Accessed April 19, 2020.
- Cohen AJ, Weinberg DA. Evaluation and Management of Blepharoptosis. New York, NY: Springer New York; 2011.
- Garg A, Alió Jorge L. Surgical Techniques in Ophthalmology: Oculoplasty and Reconstructive Surgery. New Delhi: Jaypee Brothers Medical Publishers; 2010.
- Kanski JJ, Bowling B. Clinical Ophthalmology: a Systematic Approach. Edinburgh: Elsevier; 2012.
- Watanabe A, Araki B, Noso K, Kakizaki H, Kinoshita S. Histopathology of Blepharoptosis Induced by Prolonged Hard Contact Lens Wear. American Journal of Ophthalmology. 2006;141(6). doi:10.1016/j.ajo.2006.01.032.
- Yanoff M, Duker JS. Ophthalmology. St. Louis: Mosby; 2009.

