Anterior Segment Optical Coherence Tomography Features of Keratitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Optical coherence tomography (OCT) was initially developed for imaging and analyzing the macula and optic nerve, but it is also being utilized to image the ocular surface and anterior segment of the eye.[1] Anterior segment OCT (AS-OCT) produces high-resolution cross-sectional images in a quick and noninvasive manner creating an “optical biopsy” of various anterior segment structures.[1] AS-OCT provides information about structural changes predominantly: infiltrates and scars appear as hyper-reflective lesions within the corneal stroma, inflammatory plaques as hyper-reflective lesions over the endothelium or epithelium, corneal edema as increase in corneal thickness, and stromal necrosis as cystic spaces within the stroma.
Peripheral Ulcerative Keratitis
Peripheral ulcerative keratitis (PUK) comprises a group of inflammatory diseases that cause corneal epithelial ulceration and then stromal destruction leading to peripheral corneal thinning.[2] PUK is associated with many systemic inflammatory disorders such as rheumatoid arthritis, polyarteritis nodosa, inflammatory bowel disease, systemic lupus erythematosus, and anti-neutrophil cytoplasmic antibodies (ANCA) vasculitides,[3] but can also occur secondary to infectious processes or as an idiopathic phenomenon.
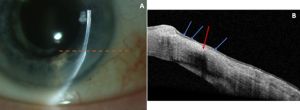
AS- OCT has been used to monitor disease activity and to categorize PUK into three stages: 1) acute stage, 2) healing stage, and 3) healed stage.[2] On AS-OCT, the acute stage is characterized by an absent corneal epithelium and disorganized anterior stroma with heterogeneous stromal reflectivity and focal thinning.[2] The healing stage demonstrates a hyporeflective irregular epithelium and less heterogeneous hyperreflective stroma.[2] Finally, the healed stage reveals a hyporeflective irregular epithelium, a demarcation line between the hyporeflective epithelium and hyperreflective stroma, and irregular thickening.[2]
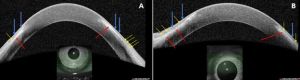
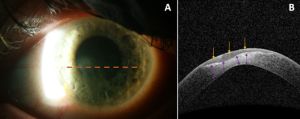
AS-OCT can also be used to monitor the stabilization of corneal thinning with appropriate treatment.[4] Figure 1A depicts a slit lamp photograph and Figure 1B an AS-OCT image of a patient in the healing stage of PUK with the hyporeflective and irregular epithelium along with moderately heterogeneous stroma. The AS-OCT images in Figure 2 demonstrate characteristic findings in the healed stage of PUK: a thinned area of hyporeflective epithelium overlying heterogenous hyperreflective stroma with a sharp line of demarcation between these two areas.
Non-Infectious Interstitial Keratitis
Interstitial keratitis is a non-ulcerative, non-suppurative, inflammatory reaction of the corneal stroma with or without associated neovascularization.[5] Also called stromal keratitis, this can be associated with localized or systemic infectious or non-infectious processes and is characterized by cellular and vascular infiltration of the stroma primarily with occasional secondary involvement of the corneal epithelium or endothelium.[5] The end result of interstitial keratitis is stromal disorganization which can lead to scar formation and reduced visual acuity from media opacity or induced astigmatism. Severe thinning can also be associated with a risk of spontaneous corneal perforation.
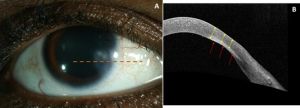
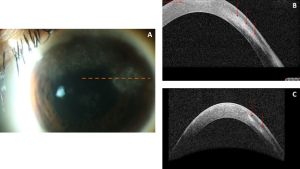
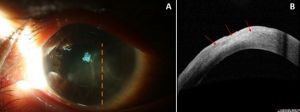
AS-OCT can be used to monitor treatment response as well as corneal thickness in areas of stromal keratitis.[6] Figure 3 demonstrates non-infectious interstitial keratitis of the same eye initially and then three months after treatment with topical prednisolone and systemic methotrexate. Figure 4 demonstrates the sequelae of interstitial keratitis–stromal thinning and a stromal scar.
Infectious Interstitial Keratitis
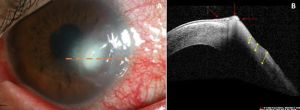
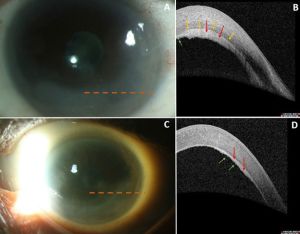
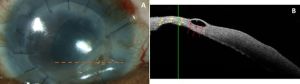
Infectious keratitis can also cause vision loss via similar mechanisms as seen in non-infectious keratitis and can have viral, bacterial, fungal, or parasitic etiologies.[7] Risk factors for infectious keratitis include contact lens wear, trauma, and severe ocular surface disease.[6] Microbial keratitis is usually diagnosed based on a history of an inciting microtrauma to the epithelium and slit lamp examination identifying a corneal epithelial defect overlying a focal stromal infiltrate. AS-OCT can be used to monitor resolution or clinical decline in patients with infectious stromal keratitis. Figure 5 demonstrates active infectious keratitis with anterior stromal and epithelial hyperreflectivity and thinning. Figure 6 demonstrates bacterial keratitis adjacent to an area of prior stromal thinning. Figure 7 shows the sequelae of microbial keratitis, stromal thinning with a thickened epithelium.
The microstructure changes in active herpetic stromal keratitis (HSK) and inactive HSK have been demonstrated on AS-OCT as epithelial and stromal thickening and epithelial and stromal changes due to corneal scarring, respectively.[8]
Endothelial Plaques
AS-OCT can highlight endothelial pathology which may be a sequela of uveitis in the setting of keratitis. Figure 8 demonstrates an endothelial plaque with a clear boundary between the cornea and plaque. This suggests that plaque formation is from an intense inflammatory reaction resulting in a cluster of inflammatory material from the anterior chamber depositing on the corneal endothelium.[9]
Corneal Thinning/Perforation
Particularly in corneas with dense infiltrates and significant corneal edema, the depth of involvement may be difficult to ascertain on slit lamp biomicrosopy. AS-OCT allows for the monitoring of the depth of corneal involvement and corneal thickness. For instance, in fungal keratitis, AS-OCT have been demonstrated to help detect satellite lesions and necrotic spaces.[10]
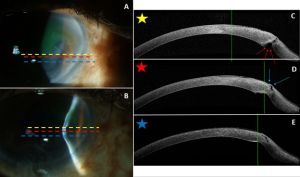
Corneal perforations are ocular emergencies that can occur secondary to penetrating trauma in healthy corneas or mild insults in those with active or prior keratitis with associated stromal thinning. These patients may also have shallow/flat anterior chambers with aqueous egress (Seidel positivity). While detectable by slit lamp examination in most cases, AS-OCT can be used to assess areas which may be suspicious or difficult to view through areas of corneal haze with stromal thinning. Figure 9 demonstrates corneal thinning in a patient who had a prior penetrating keratoplasty for corneal perforation. AS-OCT shows a sub-epithelial cystoid change. Figure 10 depicts an area of ectasia found to be Seidel positive. The corresponding AS-OCT image highlights the posterior corneal ectasia with a focal tract and excavation leading to the ocular surface.
Conclusion
AS-OCT is becoming increasingly utilized for various ocular surface and anterior segment pathologies. More investigation can further characterize additional AS-OCT findings and better inform clinical decision-making.
- ↑ 1.0 1.1 Venkateswaran N, Galor A, Wang J, Karp CL. Optical coherence tomography for ocular surface and corneal diseases: a review. Eye Vis (Lond). 2018;5:13. doi:10.1186/s40662-018-0107-0
- ↑ 2.0 2.1 2.2 2.3 2.4 Bonnet C, Debillon L, Al-Hashimi S, et al. Anterior segment optical coherence tomography imaging in peripheral ulcerative keratitis, a corneal structural description. BMC Ophthalmol. May 25 2020;20(1):205. doi:10.1186/s12886-020-01466-1
- ↑ Fu L, Jones S. Peripheral Ulcerative Keratitis. [Updated 2021 Oct 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574556/
- ↑ Garg A, De Rojas J, Mathews P, et al. Using Anterior Segment Optical Coherence Tomography to Monitor Disease Progression in Peripheral Ulcerative Keratitis. Case Rep Ophthalmol Med. 2018;2018:3705753. doi:10.1155/2018/3705753
- ↑ 5.0 5.1 Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
- ↑ 6.0 6.1 Konstantopoulos A, Kuo J, Anderson D, Hossain P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am J Ophthalmol. 2008;146(4):534-542. doi:10.1016/j.ajo.2008.05.030
- ↑ Durand ML, Barshak MB, Chodosh J. Infectious Keratitis in 2021. JAMA. 2021;326(13):1319-1320. doi:10.1001/jama.2021.0424
- ↑ Rodriguez-Garcia A, Alfaro-Rangel R, Bustamante-Arias A, Hernandez-Camarena JC. In Vivo Corneal Microstructural Changes in Herpetic Stromal Keratitis: A Spectral Domain Optical Coherence Tomography Analysis. J Ophthalmic Vis Res. Jul-Sep 2020;15(3):279-288. doi:10.18502/jovr.v15i3.7446
- ↑ Takezawa Y, Suzuki T, Shiraishi A. Observation of Retrocorneal Plaques in Patients With Infectious Keratitis Using Anterior Segment Optical Coherence Tomography. Cornea. 2017;36(10):1237-1242. doi:10.1097/ICO.0000000000001286
- ↑ Sharma N, Singhal D, Maharana PK, et al. Spectral Domain Anterior Segment Optical Coherence Tomography in Fungal Keratitis. Cornea. Nov 2018;37(11):1388-1394. doi:10.1097/ICO.0000000000001715

