Amiodarone Associated Optic Neuropathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Amiodarone is a di-iodinated benzofuran derivative developed for the treatment of angina pectoris in the 1960s.[1] By 2000, it had become the most commonly prescribed antiarrhythmic drug in North America with over 400,000 individuals receiving amiodarone for the treatment of atrial fibrillation alone.[2] Common systemic side effects of amiodarone include thyroid dysfunction, pulmonary toxicity, drug interactions (especially with other cardiac medications), peripheral neuropathy, ataxia, photosensitivity, and gastrointestinal problems[3], with Amiodarone-associated hypersensitivity pneumonitis being a possibly fatal complication, if amiodarone long-term use.
Disease Entity
Amiodarone (Cordarone, Nextrone, Pacerone) has been documented to cause ocular toxicity. The most common being corneal microdeposits known as verticillate keratopathy occurring in 70-100% of patients. The most serious side effect is optic nerve injury, with unilateral or bilateral visual loss that can rarely progress to permanent blindness. Other ocular side effects noted, which rarely cause visual impairment, include: anterior subcapsular lens opacities, multiple chalazia, dry eye syndrome[3]; photosensitivity, loss of facial hair -eyelashes or optic nerve edema and scotoma, among the ones reported[4]
Disease
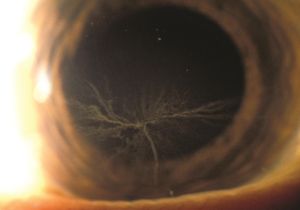
Amiodarone associated optic neuropathy (AAON) has been characterized by insidious onset with protracted disc swelling which may initially present unilaterally but subsequently involves the fellow eye as expected with systemic toxicity.[2] The mean duration of amiodarone use before visual loss was 9 months and optic nerve swelling frequently persists for several months and resolves much slower than seen in typical non-arteritic anterior ischemic optic neuropathy (NAION).[1] [2] Given the potential progressive deterioration of vision from the optic neuropathy, cessation of amiodarone or dose reduction may be considered for patients who develop optic neuropathy and this should be coordinated with their cardiologist.[5]
If NAION is suspected, atypical features that may point towards AAON include insidious onset, contralateral optic nerve large cup, mild optic nerve dysfunction (visual field, a visual acuity ≥20/40, relative afferent pupillary defect [RAPD] of ≤0.9 log units), and persistent duration of disc edema[6]
Risk Factors
The relationship between amiodarone and optic neuropathy has been controversial as clinical features of AAON is quite similar to NAION and many patients taking amiodarone often have cardiovascular risk factors and are also at high risk of developing NAION.[1] However, AAON differs from NAION in that AAON occurs more often in men and with systemic hypertension, while NAION has equal sex predilection. In a review of 296 cases of AAON, mean patient age was 66 years with 74% male. The mean duration of amiodarone therapy before development of visual symptoms was 9 months; however, the range was 1 to 84 months. This demonstrate that uniformity in the timing between initiation of amiodarone therapy and AAON has not been established. The median dose of patients presenting with visual symptoms was 200 mg/day and the range was 57 to 1,200 mg/day.[2] Additionally, digitalis was found to be the most frequent concurrent drug in AAON patients.[2]
Pathophysiology
Ultrastructural findings of the optic nerve in patients with AAON demonstrate selective accumulation of intracytoplasmic lamellar inclusions in the large axons of the optic nerve. Accumulation of lamellated bodies has been attributed to inhibition of lysosomal sphingomyelinases suggesting primary lipidosis which may mechanically or biochemically decrease axoplasmic flow as the likely mechanism of optic nerve damage in AAON.[2][7] Such a mechanism is similar to those seen in patients with amiodarone-induced peripheral neuropathy in which demyelination and lamellated bodies are seen in the axoplasm and Schwann cells of peripheral nerves.[2] A recent study on rats in 2017 postulated the reason why amiodarone affects the retinal neuronal cell layers: apoptosis of retinal ganglion cells (RGC) with resultant a and b wave decreased in electroretinogram. Insulin-like growth factor-1 (IGF-1) showed to reverse this effect[8] [9]
In some cases edema resolves after stoping amiodarone, demonstrated by resolution of blind spot on automated VF in 7months and resolution of edema by OCT readings in 15months[10]
In contrast, some cases may never show edema and even have normal-to-large CDR (not a "disc at risk") and develop only visual acuity and visual field loss that improves after stopping Amiodarone[11]
Primary Prevention
Due to the use of amiodarone in the treatment of life-threatening ventricular and supraventricular arrhythmias, discontinuation should not be taken lightly and needs to always be discussed with cardiology.[12] When amiodarone therapy presents with corneal microdeposits (i.e., vortex keratopathy, Figure 1), the most common ocular side effect, patients can usually continue amiodarone therapy. Cessation of the drug or dose reduction might however be considered for patients with AAON after the risk-benefit of continuing the drug vs worsening of cardiac condition or considering changing to an alternate drug has been discussed with the cardiologist, ophthalmologist and the patient.[5] Because most cases of AAON occur within 12 months of drug initiation, equally spaced interval evaluation within the first year followed by annual examination has been suggested.[13] In a review article it was noted that over half the cases of AAON had improved acuity following drug cessation, around 20% remained the same and another 20% had worsening vision.[2] Unfortunately, there is no screening protocol or defined risk stratification strategy for AAON.
In a retrospective systematic review of a small dose, considered 100mg a day, only showed the incidence of side effects of 0.17 [95% confidence interval (CI): 0.12–0.22][14].
Diagnosis
History
The severity of visual loss is variable in patients with AAON, ranging from mild reversible visual loss to severe permanent blindness. In the 296 AAON patients reviewed by Passman et al., 44% had insidious onset with nearly one third being asymptomatic.[2] Patients with AAON presenting with ocular symptoms reported either acute (19%) or insidious (26%) monocular vision loss or acute bilateral (10%) or insidious (14%) visual loss.
Physical Examination
Optic disc edema was reported in 85% of cases with two-thirds of AAON cases presenting with bilateral simultaneous optic neuropathy.[2] While the optic disc edema of AAON can resemble that of NAION, the median duration of optic disc edema in AAON after cessation of amiodarone use is 3 months, but it may take up to 15mo in some cases.[10] Finally, whereas NAION occurs most often in patients with small optic nerve cup-disc ratio, there is no particular cup-disc ratio associated with AAON.[2] Some authors have suggested to look for “atypical” features of NAION in order to identify AAON such as other systemic adverse effects of the medication, contralateral eye large cup, degree of dysfunction and onset). It is also possible to find sequential optic disc swelling.[8] Corneal verticillate is frequently seen concomitant to optic nerve toxicity.
Signs
AAON should be suspected in patients taking amiodarone presenting with insidious onset, slow progression, protracted disc swelling leading to unilateral or bilateral visual loss, and visual findings that require several months to stabilize after cessation of the drug. Although some cases may present unilaterally, subsequent development of involvement of the fellow eye is seen in most cases as expected with systemic toxicity.[2]
Symptoms
Patients AAON may present with monocular or bilateral decreased visual acuity occurring acutely or insidiously. Up to a third of patients with AAON may be asymptomatic. Other symptoms may include dyschromatopsia, and nerve fiber type visual field defects.[3]
Systemic symptoms, also adverse effects of Amiodarone include: further arrhythmia (torsade de point), hypotension (when IV), angioedema, rash or anaphylactic reactions.
Diagnostic procedures
Fluorescein angiography will often reveal swollen optic discs frequently bilaterally (even if clinically unilateral) with possible overlying hemorrhage. Humphrey visual field testing has demonstrated visual field defects ranging from superior or inferior hemifield defect to central scotoma.[15] Ishihara pseudoisochromatic colour plates test can be conducted as some patients have reported dyschromatopsia in certain cases.[3] Unilateral or bilateral but asymmetric cases will have a relative afferent pupillary defect (RAPD).
Laboratory test
There are no indicated laboratory tests. Neuroimaging studies are normal and do not show enhancement of the optic nerve.
Differential diagnosis
NAION presents similarly to AAON and is characterized by acute, unilateral disc swelling with hemorrhage which commonly resolves within 2 to 6 weeks.[1] Although AAON share features of NAION, optic swelling in AAON can persist up to around 8-15 months after amiodarone cessation. Additionally, patients with NAION also tend to have small, crowded optic discs, which may not be the case in patients with AAON.[1]
Management
General treatment
At the first signs of optic toxicity, patent should consult the cardiologist regarding the feasibility of discontinuing the drug with alternative medication or catheter ablation, and leave the decision regarding the safety of discontinuing amiodarone to them.[1]
Some cardiology groups offer guidance on preventive follow ups with eye examination at baseline and frequent follow ups if underlying abnormality; if preexisting optic nerve pathology/neuritis some recommend to avoid medication[16]
Medical therapy
No medical therapy is currently indicated for the treatment of AAON. Patients with onset of visual symptoms would have ophthalmologic examination expeditiously and discontinuing or dose reduction of amiodarone might be considered.[3] As mentioned before, some studies in animals suggest that IGF-1 therapy reversed the effects of amiodarone in GCL in rats[8], it is unknown if this will play a role in the future in humans.
Medical follow up
When ocular toxicity is suspected, physicians should report ophthalmic findings, concomitant drugs, drug dose, and duration of amiodarone therapy, and follow-up findings to the FDA’s MedWatch program. Patients should be alerted to the need for baseline and regular follow-up ophthalmologic screening.[2]
Surgery
There is no surgical therapy.
Prognosis
Following drug cessation, 58% experienced improvement of vision, 21% were unchanged, and 21% had further decrease in visual acuity. Legal blindness (<20/200) was noted in at least one eye in 20% of cases. Given the risk of permanent vision loss, close ophthalmic surveillance of patients during the tenure of amiodarone administration may be warranted.[2]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 Wang AG, Cheng HC. Amiodarone-Associated Optic neuropathy: Clinical Review. Neuroophthalmology. 2016;41(1):55-58.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Passman RS, Bennett CL, Purpura JM, et al. Amiodarone-Associated Optic neuropathy: A Critical Review. Am J Med. 2012;125(5):447-453.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Nagra PK, Foroozan R, Savino PJ, et al. Amiodarone induced optic neuropathy. Br J Ophthalmol. 2003. 87:420-422.
- ↑ Colunga Biancatelli RM, Congedo V, Calvosa L, Ciacciarelli M, Polidoro A, Iuliano L. Adverse reactions of Amiodarone. J Geriatr Cardiol. 2019 Jul;16(7):552-566. doi: 10.11909/j.issn.1671-5411.2019.07.004. PMID: 31447894; PMCID: PMC6689516.
- ↑ Jump up to: 5.0 5.1 Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998. 42(4):360.
- ↑ Mitchell R, Chacko J. Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy. Biomolecules. 2022;12(9):1298. Published 2022 Sep 14. doi:10.3390/biom12091298
- ↑ Mansour AM, Puklin JE, O’Grady R. Optic nerve ultrastructure following amiodarone therapy. J Clin Neuroophthalmol. 1998. 8(4):231-7.
- ↑ Jump up to: 8.0 8.1 8.2 Liao R, Yan F, Zeng Z, Farhan M, Little P, Quirion R, Srivastava L, Zheng W. Amiodarone-induced retinal neuronal cell apoptosis attenuated by IGF-1 via counter regulation of the PI3k/Akt/FoxO3a pathway. Mol Neurobiol 2016;65:294–213
- ↑ Fasler K, Traber GL, Jaggi GP, Landau K. Amiodarone-associated Optic Neuropathy-A Clinical Criteria-based Diagnosis?. Neuroophthalmology. 2017;42(1):2‐10. Published 2017 Aug 18. doi:10.1080/01658107.2017.1340961
- ↑ Jump up to: 10.0 10.1 Shinder, Roman MD; Frohman, Larry P MD; Turbin, Roger E MD, FACS Regression of Bilateral Optic Disc Edema After Discontinuation of Amiodarone, Journal of Neuro-Ophthalmology: September 2006 - Volume 26 - Issue 3 - p 192-194 doi: 10.1097/01.wno.0000235581.02922.6c
- ↑ Speicher, Matthew A. MD; Goldman, Michael H. MD, FACC; Chrousos, Georgia A. MD Amiodarone Optic Neuropathy Without Disc Edema, Journal of Neuro-Ophthalmology: September 2000 - Volume 20 - Issue 3 - p 171-172
- ↑ Lee AG, Rouleau J, Longmuir R. Controversies in Neuro-Ophthalmology. CRC Press, 2016, pp. 74-78.
- ↑ Johnson LN, Krohel GB, Thomas ER. The clinical spectrum of amiodarone-associated optic neuropathy. J Natl Med Assoc. 2004;96(11):1477-91.
- ↑ Chokesuwattanaskul R, Shah N, Chokesuwattanaskul S, Liu Z, Thakur R. Low-dose Amiodarone Is Safe: A Systematic Review and Meta-analysis. J Innov Card Rhythm Manag. 2020;11(4):4054-4061. Published 2020 Apr 15. doi:10.19102/icrm.2020.110403
- ↑ Purvin V, Kawasaki A, Borruat FX. Optic Neuropathy in Patients Using Amiodarone. Arch Ophthalmol. 2006;124(5):696-701.
- ↑ Merino JL, Perez de Isla L. Treatment with amiodarone: how to avoid complications:An article from the e-Journal of Cardiology Practice. Vol. 10, N° 2 - 20 Sep 2011,https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-10/Treatment-with-amiodarone-How-to-avoid-complications, accessed 9/23/24