Alagille Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Alagille Syndrome is an autosomal-dominant inherited disease caused by mutations in the JAG1 and NOTCH2 genes.[1] First described by its characteristic intrahepatic bile duct hypoplasia, Alagille Syndrome is now known to impact multiple organ systems[2]. Those that are most commonly affected are the hepatobiliary, oral maxillofacial, ophthalmic, cardiovascular, renal and musculoskeletal[2]. The estimated prevalence of Alagille syndrome ranges from 1:30,000 to 1:100,000[3]. Variable disease presentation is thought to be due to variable penetrance, with many family members of those diagnosed having characteristics but not meeting clinical diagnostic criteria.[2][4][5]
Pathogenesis
Mutations are much more common in JAG1, with very few cases being caused by mutation of the NOTCH2 gene.[6] These genes function in development to aide in cell differentiation in utero.[7] Thus, it is believed that mutations affecting these genes may disrupt normal differentiation and development of the many organ systems affected.
Systemic presentation
There are seven major clinical features of Alagille syndrome:
- Cardiac defects
- Hepatic abnormalities
- Renal dysfunction
- Skeletal abnormalities
- Ophthalmic findings
- Facial features
- Vascular anomalies
Cardiac involvement is reported in more than 90% of patients[8], with presentations ranging from benign murmur to structural defects requiring surgical intervention.[5] Reported cardiac anomalies include: peripheral pulmonic stenosis, tetralogy of Fallot, ventricular septal defect, atrial septal defect, and coarctation of the aorta[8]. Right-sided heart defects are more common than left in these patients.[9] More severe defects in cardiac structure correlate with greater morbidity and mortality.[5]
The liver is the most severely affected organ in Alagille syndrome, and hepatobiliary symptoms are what tend to guide the primary physician to investigate further. Parents may notice their child developing jaundice, pruritis, and cutaneous xanthomas. Patients may also develop coagulopathy as a consequence of poor hepatic function and decreased bile-assisted digestion of fat-soluble vitamins. Upon investigation, the inspecting physician may find an indirect bilirubinemia, hepatomegaly, and imaging suggestive of biliary atresia.[10] As the disease progresses, patients may require liver transplant due to cholestasis, or due to the development of portal hypertension and fibrosis.[5][3] A total bilirubin greater than 3.8mg/dL between ages of 12-24 months, as well as the presence of xanthomas and liver fibrosis before the age of 5 years correlate with a worse prognosis.[11] Up to 15% of patients will develop end-stage liver disease[8]. Fifteen–30% of children with hepatic involvement will progress to requiring a liver transplantation by age 5 years.[12]
Renal involvement may include renal tubular acidosis, vesicoureteral reflux, glomerular mesangiolipidosis and renal dysplasia.[13][8]
In terms of skeletal manifestations of the disease, patients may present with fractures and clinical signs of osteopenia. The most frequent musculoskeletal abnormality in Alagille Syndrome is a butterfly abnormality of the vertebral column.[14]
Facial features are notable for a saddle-like nose, deep-set eyes with hypertelorism, prominent ears, triangular face with a pointed chin and a high forehead with frontal bossing or flattening.[4][8]
Patients are more likely to have cerebral vessel anomalies like aneurysms and Moyamoya syndrome[8], but many vessels extracranially can be affected by Alagille Syndrome. Although pulmonary vessels are commonly involved in disease, the renal vascular system and middle aorta may also be affected.
Mouse model studies suggest that those with Alagille syndrome may also be at risk for hearing loss.[15]
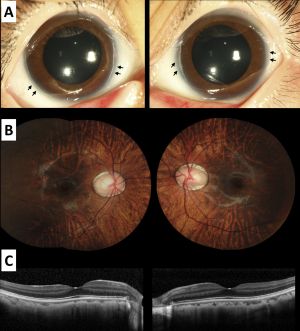
Ophthalmic presentation
The most common ocular manifestation in patients with Alagille Syndrome is posterior embryotoxon. Anterior segment ocular involvement may also include iris abnormalities, but intraocular pressures and pupillary function tend to be normal. Posterior segment abnormalities may include pathologic changes to the optic disc including hypoplasia, elevation, and irregular tilting of the disc. Chorioretinal findings may include diffuse hypopigmentation of the choroid and retina, and irregular pigmentation of the retinal pigment epithelium. Recurrent intraretinal hemorrhages have also been reported, suggesting a pathological microvascular source.[16] Even with these various ocular findings associated with Alagille Syndrome, visual function in these patients tends to be unaffected.[17] Although, case series data suggests that some patients with Alagille Syndrome may suffer visually significant macular atrophy.[18]
Differential Diagnosis
Main considerations in the differential diagnosis of Alagille involve diseases that lead to cholestasis. However one could consider a differential for each organ system affected.
- Bile duct paucity: alpha-1 antitrypsin deficiency, cystic fibrosis, childhood primary sclerosing cholangitis, mitochondrial disorders, congenital hepatic fibrosis, infectious etiologies (congenital syphilis, congenital cytomegalovirus, congenital rubella, hepatitis B), childhood autoimmune hepatitis, hypopituitarism, graft versus host disease, Zellweger syndrome, Ivemark syndrome, Smith-Lemli-Opitz syndrome
- Cholestasis: biliary atresia, sepsis, galactosemia, tyrosinemia, choledochal cyst, progressive familial intrahepatic cholestasis types 1 and 2, arthrogryposis-renal dysfunction-cholestasis syndrome, benign recurrent intrahepatic cholestasis, Norwegian cholestasis (also called Aagenaes syndrome)
- Pulmonary vessel anomalies: Noonan syndrome, Watson syndrome, William syndrome, Down syndrome, LEOPARD syndrome
- Cardiac and skeletal malformations: deletion 22q11.2
- Posterior embryotoxin: Bannayan-Riley-Ruvalcaba syndrome, Axenfeld-Rieger syndrome. Also seen in 8-15% of the general population.
Diagnosis
Diagnosis without a confirmed genetic test can be deduced by the characteristic inclusion of at least three of the seven major clinical features. Patients with clinically suspected Alagille Syndrome are also able to undergo genetic testing for mutations in the JAG1 and/or NOTCH2 genes for confirmation. Up to 95% of patients with Alagille Syndrome have an identifiable mutation that will confirm the diagnosis.[19] In cases of pregnancy with a family history, prenatal and preimplantation testing may be available. If both genetic panels come back negative for mutations, findings should be clinically correlated with other possible diagnoses. Previously, diagnosis was confirmed with liver biopsy, with the sparsity of bile ducts being the correlating finding for confirmation.
Management
Treatment of Alagille Syndrome is mainly geared toward managing the dysfunction of the affected organ systems. Patients often present with symptoms related to their lack of a mature hepatobiliary system, including pruritis, jaundice, right upper quadrant pain, and malabsorption. To manage this hepatobiliary dysfunction, patients are advised to ingest calorie-dense food and supplement fat-soluble vitamins to ensure proper overall absorption of nutrients.[20] Medical treatment focuses primarily on increased bile secretion into the intestines. Ursodeoxycholic acid (UDCA), rifampin, cholestyramine, opioid antagonists, antihistamines and selective serotonin re-uptake inhibitors may all be trialed. UDCA has been the first-line medication prescribed for pruritis in these patients.[21] Newer medical treatments include the ileal bile acid transport inhibitors maralixibat and odevixibat. Maralixibat is FDA approved for patients as young as 3 months and odevixibat later as young 12 months. Ongoing studies suggest this new class of medical therapies may be associated with improved event-free survival and quality of life.[22] Some are considering IBAT inhibitors as first-line treatments for those with Alagille Syndrome given their effectiveness and safety profile.[23] Furthermore, early use of IBAT inhibitors shows promise in reducing the need for liver transplant in patients with Alagille.[24] Patients that fail medical therapy may opt for partial external biliary diversion as surgical intervention.[25]
Cardiac, renal and vascular issues may be managed medically or surgically depending on their disease severity. Ophthalmic and skeletal disease rarely requires intervention. However, due to the variability in disease manifestation, coordination of a multisubspecialty team of providers is often necessary for diagnosis and surveillance of disease.[26]
Prognosis
Prognosis varies depending on disease severity. Up to 75% of patients require liver transplantation by 18 years of age.[27] Cerebrovascular events are a significant cause of morbidity and mortality.[28] Visual prognosis tends to be favorable.
- ↑ Krantz ID, Colliton RP, Genin A, et al. Spectrum and frequency of jagged1 (JAG1) mutations in Alagille syndrome patients and their families. Am J Hum Genet. 1998;62:1361–1369.
- ↑ 2.0 2.1 2.2 Alagille D, Estrada A, Hadchouel M, Gautler M, et al. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): Review of 80 cases. J. Pediatr. 1987, 110, 195–200.
- ↑ 3.0 3.1 Lykavieris P, Hadchouel M, Chardot C, Bernard O. Outcome of liver disease in children with Alagille syndrome: A study of 163 patients. Gut. 2001, 49, 431–435.
- ↑ 4.0 4.1 Saleh M, Kamath BM, Chitayat D. Alagille syndrome: Clinical perspectives. Appl. Clin. Genet. 2016, 9, 75.
- ↑ 5.0 5.1 5.2 5.3 Emerick KM, Rand EB, Goldmuntz E, Krantz ID, et al. Features of Alagille syndrome in 92 patients: Frequency and relation to prognosis. Hepatology 1999, 29, 822–829.
- ↑ Gilbert MA, Bauer RC, Rajagopalan R, Grochowski CM, et al. Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification. Hum. Mutat. 2019, 40, 2197–2220.
- ↑ Kamath B, Bason L, Piccoli D, Krantz I, Spinner N. Consequences of JAG1 mutations. J. Med. Genet. 2003, 40, 891–895.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Diaz-Frias J, Kondamudi NP. Alagille Syndrome. 2021 Nov 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 29939604.
- ↑ McElhinney DB, Krantz ID, Bason L, Piccoli DA, et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 2002, 106, 2567–2574.
- ↑ Subramaniam P, Knisely A, Portmann B, Qureshi S, et al. Diagnosis of Alagille syndrome—25 years of experience at King’s College Hospital. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 84–89.
- ↑ Mouzaki M, Bass LM, Sokol RJ, Piccoli DA, Quammie C, Kamath BM. Early life predictive markers of liver disease outcome in an International, Multicentre Cohort of children with Alagille syndrome. Liver Int. 2015 Jul 22; Epub.
- ↑ Kamath BM, Loomes KM, Piccoli DA. Medical management of Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010 Jun;50(6):580-6.
- ↑ Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A. 2012;158A:85–89.
- ↑ Sanderson E, Newman V, Haigh SF, Baker A, Sidhu PS. Vertebral anomalies in children with Alagille syndrome: an analysis of 50 consecutive patients. Pediatr Radiol. 2002;32:114–119.
- ↑ de Haan S, Corbat AA, Cederroth CR, Autrum LG, Hankeova S, Driver EC, Canlon B, Kelley MW, Andersson ER. Jag1 represses Notch activation in lateral supporting cells and inhibits an outer hair cell fate in the medial cochlea. Development. 2024 Oct 7:dev.202949.
- ↑ Law C, Pattathil N, Simpson H, Ward MJ, Lampen S, Kamath B, Aleman TS. Intraretinal hemorrhages and detailed retinal phenotype of three patients with Alagille syndrome. Ophthalmic Genet. 2024 Oct;45(5):522-531.
- ↑ Hingorani M, Nischal KK, Davies A, Bentley C, et al. Ocular abnormalities in Alagille syndrome. Ophthalmology 1999, 106, 330–337.
- ↑ Paez-Escamilla M, Scanga HL, Liasis A, Nischal KK. Macular atrophy in JAG1-related Alagille syndrome: a case series. Ophthalmic Genet. 2022 Apr;43(2):230-234.
- ↑ Gilbert MA, Bauer RC, Rajagopalan R, Grochowski CM, et al. Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification. Hum. Mutat. 2019, 40, 2197–2220.
- ↑ Ayoub MD, Kamath BM. Alagille Syndrome: Diagnostic Challenges and Advances in Management. Diagnostics. 2020; 10(11):907.
- ↑ Kronsten V, Fitzpatrick E, Baker A. Management of cholestatic pruritus in paediatric patients with alagille syndrome: The King’s College Hospital experience. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 149–154.
- ↑ Spinner NB, Loomes KM, Krantz ID, et al. Alagille Syndrome. 2000 May 19 [Updated 2024 Jan 4]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024.
- ↑ Jarasvaraparn C, Rodrigo M, Hartley C, Karnsakul W. Exploring odevixibat's efficacy in alagille syndrome: insights from recent clinical trials and IBAT inhibitor experiences. Expert Opin Pharmacother. 2024 Aug;25(12):1647-1655. doi: 10.
- ↑ Jankowska I, Socha P, Gliwicz D, Lipiński P, Rokicki D, Kaliciński P, Danielewska E, Grenda R. Modified by the Innovative Drugs and Strategies-Pattern of Selected Indications for Pediatric Liver Transplantation. Pediatr Transplant. 2024 Aug;28(5):e14825.
- ↑ Wang KS, Tiao G, Bass LM, Hertel PM, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology 2017, 65, 1645–1654.
- ↑ Menon J, Shanmugam N, Vij M, Rammohan A, Rela M. Multidisciplinary Management of Alagille Syndrome. J Multidiscip Healthc. 2022 Feb 23;15:353-364.
- ↑ Kamath BM, Ye W, Goodrich NP, Loomes KM, et al. Outcomes of Childhood Cholestasis in Alagille Syndrome: Results of a Multicenter Observational Study. Hepatol. Commun. 2020, 4, 387–398.
- ↑ Kamath BM, Spinner NB, Emerick KM, Chudley AE, et al. Vascular anomalies in Alagille syndrome: A significant cause of morbidity and mortality. Circulation 2004, 109, 1354–1358.

