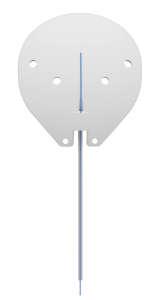
Ahmed ClearPath Glaucoma Drainage Device
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Glaucoma drainage devices (GDDs) play an important role in the surgical management of different types of pediatric and adult glaucoma. GDDs are classified into valveless GDDs including Baerveldt glaucoma implant or valved GDDs including Ahmed glaucoma valve.[1][2] Ahmed ClearPath (New World Medical, Rancho Cucamonga, CA) is a novel valveless GDD that was approved in 2019 by the US Food and Drug Administration and was introduced in the market for the management of glaucoma. A recent survey of the American Glaucoma Society found that 17% of respondents preferred the Ahmed Clearpath as a non-valved aqueous shunt device.[3]
Device
The Ahmed ClearPath design has many unique features in an attempt to make the surgical procedure technically easier with a reduced risk of complications. Ahmed ClearPath is available in two sizes: 250 mm2 (Figure 1) and 350 mm2 (Figure 2). The smaller 250 mm2 model allows for GDD placement between recti muscles without the need for muscle isolation. In the 350 mm2 models, it is still necessary to tuck it underneath the muscles, however, the winged design and the more posterior position of the plate avoid rectus muscle insertions and allow more stability of the plate when inserted.
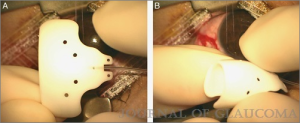
The plate is low-lying and made up of flexible material allowing easier insertion in patients with tight orbits or childhood glaucoma patients with buphthalmos and relatively small orbits (Figure 3). The anterior position of the suture fixation eyelets allows easier implant fixation without the need for extensive posterior dissection. The device is packaged with an optional pre-threaded 4/0 polypropylene ripcord suture and a 23-gauge needle which is used to create the sclerostomy. The ripcord suture is usually removed in the pediatric population because of the difficulty of removing it in the clinic postoperatively.
In a laboratory study evaluating the flow characteristics and the suture eyelet integrity of the Ahmed ClearPath 350 mm2 model compared to the Baerveldt glaucoma implant 350 mm2 model, Langenberg et al.[4] reported that the flow restriction and eyelet integrity were comparable between both devices.
Outcomes
Few studies have evaluated the safety and efficacy of Ahmed ClearPath in glaucoma management. The first series was conducted by Elhusseiny and VanderVeen[5] demonstrating their early experience of Ahmed ClearPath in seven eyes with childhood glaucoma. The median follow-up was 12 months. They reported a statistically significant reduction of the mean intraocular pressure (IOP) from 36±3.5 mmHg preoperatively to 12.4±2.8 mmHg at the last follow-up (p<0.001). There was also a statically significant reduction of the mean glaucoma medications from 2.7±0.6 medications preoperatively to 0.7±0.8 medications at the last follow-up (p=0.0009).
In adulthood glaucoma, a multicentric retrospective study by Grover et al.[6] evaluated the outcomes of Ahmed ClearPath in 104 eyes, of which 63.5% had primary open-angle glaucoma. They reported a statistically significant reduction in the mean IOP from 26.3±9 mmHg preoperatively to 13.7±4.7 mmHg at the 6-month follow-up visit (p<0.0001). The mean number of glaucoma medications was reduced from 3.9±1.3 preoperatively to 1.9±2.1 at 6-months postoperatively (p<0.0001). They reported that 91.8% of eyes had a postoperative IOP of ≤18 mmHg and 29.8% of eyes were glaucoma medication-free at the 6-month follow-up visit. The most common complications were anterior chamber inflammation (17 eyes, 16.3%), hyphema (16 eyes, 15.4%), early hypotony (7 eyes, 6.7%), and cystoid macular edema (4 eyes, 3.8%).
Conclusion
The Ahmed ClearPath is a novel non-valved GDD that effectively reduces the IOP in both childhood and adult glaucoma. Although the published literature is promising, studies were limited by small sample size, retrospective nature, and short follow-up. Further studies are needed to evaluate the long-term outcomes and safety of Ahmed ClearPath, especially in comparison to other GDDs.
References
- ↑ Shuchi Patel & Louis R. Pasquale (2010) Glaucoma Drainage Devices: A Review of the Past, Present, and Future, Seminars in Ophthalmology, 25:5-6, 265-270
- ↑ Elhusseiny AM & VanderVeen DK. Outcomes of Glaucoma Drainage Devices in Childhood Glaucoma, Seminars in Ophthalmology, 35:3, 194-204
- ↑ Zhang JY, Qiu M. Techniques and Preferences for Nonvalved Aqueous Shunts: A Survey of American Glaucoma Society Members. Ophthalmol Glaucoma. 2023 Jul 16:S2589-4196(23)00133-3. doi: 10.1016/j.ogla.2023.07.006. Epub ahead of print. PMID: 37454974.
- ↑ Langenberg K, Tran J, Koontz J, Kahook MY. Flow Resistance and Suture Eyelet Integrity of the Ahmed ClearPath Glaucoma Drainage Device. Investigative Ophthalmology & Visual Science. 2020;61(7):3142
- ↑ Elhusseiny AM, VanderVeen DK. Early Experience With Ahmed Clear Path Glaucoma Drainage Device in Childhood Glaucoma. Journal of Glaucoma. 2021;30(7).
- ↑ Grover DS, Kahook MY, Seibold LK, et al. Clinical Outcomes of Ahmed ClearPath Implantation in Glaucomatous Eyes: A Novel Valveless Glaucoma Drainage Device. J Glaucoma. 2022;31(5):335-339.