Acute Macular Neuroretinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Acute Macular Neuroretinopathy
Disease
Acute Macular Neuroretinopathy (AMN), first reported in 1975 (by Bos and Deutman)[1], is an uncommon retinal disorder, commonly affecting young women[2], due to outer retinal vascular alterations that causes transient or permanent central vision loss. Para central scotomas and decreased best-corrected visual acuity are the presenting symptoms. The diagnosis is primarily based on the structural findings seen on optical coherence tomography (OCT) imaging.
Patients with AMN report a sudden onset of one or more paracentral scotomas, decreased vision, and/or blurry vision. Clinically, intra-retinal, reddish-brown, wedge-shaped lesions are noted around the fovea. Scotomas generally persist indefinitely, though some resolve partially over months. In the majority of patients, the fovea is spared; however, prior studies have found patients with foveal involvement, including foveal hemorrhage, foveal granularity, and abnormal foveal reflex.[3]
A review article published in 2016 identified the largest series of 156 eyes of 101 patients with AMN.[4] Most cases in this study were reported in young, white females in their third decade of life. The number of unilateral versus bilateral cases were similar though there was a slightly increased percentage of bilateral AMN (55 of 101 patients, 54.4%) as compared to unilateral AMN (45 of 101 patients, 44.5%).
Recently, a few cases of AMN have been described after COVID vaccine. Fekari and co-authors in their literature review published in 2023, identified 21 such cases of which 20 (95%) [5] were women and 67% were on oral contraceptives. Majority (90%) of patients experienced visual symptoms within 8 days of the vaccine.
Etiology
Although exact mechanisms causing AMN have yet to be elucidated, there is evidence to suggest that vascular compromise of the deep retinal capillary plexus of the retina leads to the vision loss seen in patients with this disease.[3] [6] Another theory has been postulated regarding systemic inflammation in autoimmune diseases with microthrombosis causing small-vessel occlusion, leading to ischemic retinopathy.[7] This theory of microthrombosis has also been observed in several cases of COVID-19 vaccination and infection.
Risk Factors
Many associations have been identified with AMN and include:
- Infection or febrile illness (i.e. influenza, enteritis, upper respiratory tract infection, pharyngitis, bronchitis, dengue fever, etc.)
- COVID infection and vaccinations[8] [9] [5]
- Intravitreal anti-VEGF injections[10]
- Oral contraceptive pills
- Hypotension/shock due to several causes (post-partum, post-surgery, trauma etc)
- Intravenous contrast
- Intravenous ephedrine/epinephrine
- Caffeine
- Pro-thrombin associated antiphospholipid antibodies
- Pre-eclampsia
- Sinus infection
- hypercoagulation state during COVID-19 infection[11]
Pathophysiology
While the cause of AMN has not yet been identified, spectral domain-optical coherence tomography (SD-OCT) and OCT-angiography (OCT-A) have helped to elucidate upon possible mechanisms. SD-OCT has revealed hyperreflectivity in the ONL and OPL and EZ disruption. This suggests damage at the level of the photoreceptors.[12] Optical coherence tomography (OCT) shows hyper-reflectivity of the outer plexiform layer, outer nuclear layer, and disruption of ellipsoid (EZ) or interdigitation zones (IZ) with attenuation of deep capillary plexus on on OCT angiography. This is in contrast to the findings in paracentral acute middle maculopathy (PAMM), where there is attenuation of superficial capillary plexus with hyperreflectivity in the inner nuclear layer and no Z or IZ disruption.
AMN lesions have also been shown to have a topographical relationships with the choroidal watershed zone (CWZ) or patchy choroidal filling (PCF), both of which have poor vascularity.[13]
Diagnosis
Diagnostic Procedures and Tests
Funduscopic examination is often initially normal. Typically, lesions become visible 3 days to 2 months after symptom onset. Classically, retinal lesions involve one or more reddish-brown, petalloid lesions that surround the fovea. Such lesions can be subtle clinically and may be better seen with red-free light. These lesions correspond closely to Amsler grid findings of scotoma. Faint intraretinal hemorrhages can be seen.
The imaging modalities most commonly used to evaluate the lesion(s) are:
- Infrared fundus photography. SD-Optical Coherence Tomography (OCT) machines use infrared light to illuminate the macula for the photographer prior to any cross-sectional images being obtained. Lesions are visible as dark gray, petalloid, perifoveal lesions with the tip pointed toward the fovea. These correspond anatomically to the scotomas if the patient draws them on an Amsler grid or if they are documented via formal visual field testing.
- SD-OCT: Hyperreflective plaque is seen initially at outer nuclear (ONL) and outer plexiform layers of OCT, indicating disruption of photoreceptor cell bodies and their axons. Focal ellipsoid zone (EZ) disruption ensues. With time, EZ reconstitutes but persistent interdigitation zone disruption is noted. The hyper-reflective plaque fades away and is replaced with ONL thinning.
- OCT-A reveals reduced flow signals in deep retinal capillary plexus, suggesting focal ischemia photoreceptor axons in the outer plexiform layer. Variable recovery of capillary flow signal is noted with time in the deep capillary plexus. However, the entire damaged photoreceptor unit atrophies causing cumulative long-term ONL thinning.
- Fluorescein angiography is noted to be normal in most cases. Subtle hypofluorescence of the lesions may be noted early and late phases of the study.
- Adaptive Optics shows reduced cone photoreceptor density and cone mosaic disruption with incomplete recovery with time.
- Multifocal ERG reveals diminished amplitudes in most eyes and, occasionally, decreased implicit time may also be noted.
AMN Patient Images
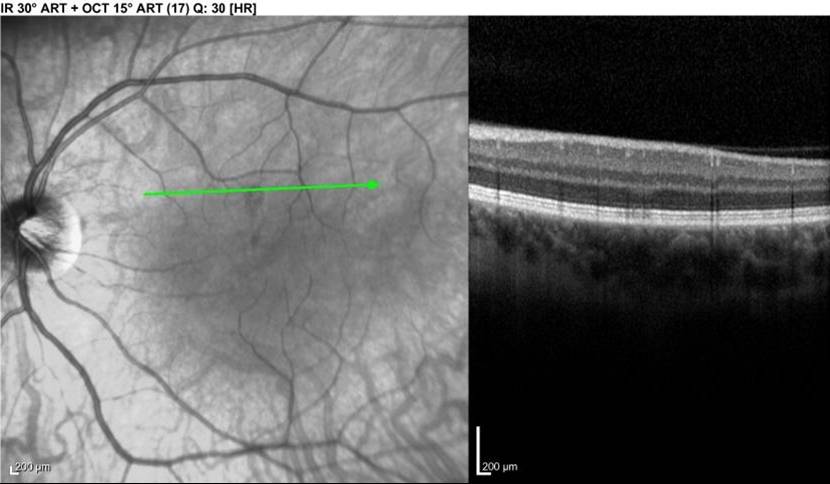
LEFT - Infrared view of left macula. Lesion is dark area at the middle of the green line. RIGHT - Focal signal reduction of the Inner Segment / Outer Segment junction within the lesion. The above lesion was NOT visible on funduscopic examination at the time of this photograph / OCT.
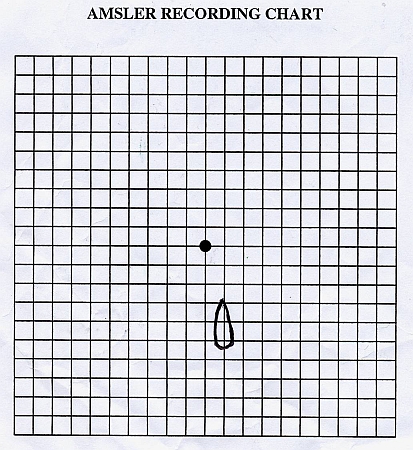
Patient's drawing of her left eye's scotoma, day 6 after symptom onset (same patient on same day as photo above)
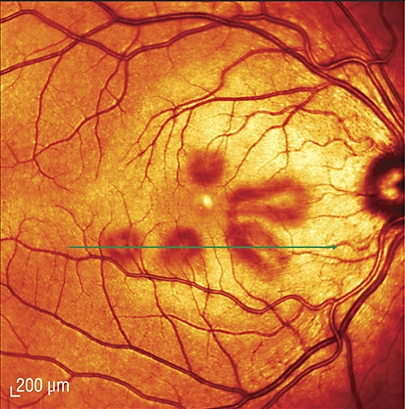
Fundoscopic examination of a patient (different than the one from above) revealing reddish-brown petalloid perifoveal lesions with the tips of the petals pointed toward the fovea
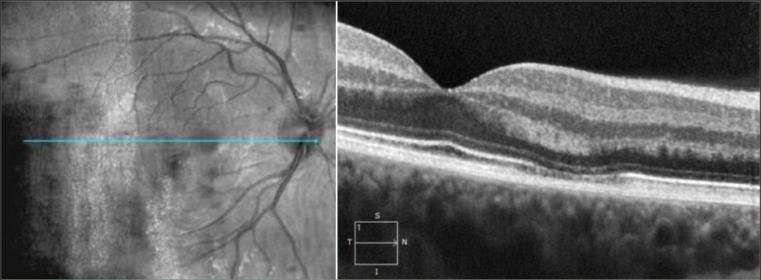
OCT image shows hyperreflective area between the outer plexiform and outer nuclear layer. Images courtesy of Dr. Stephen O'Connell
History
Patients are generally healthy women in their teens - 30's. Patients report the sudden onset of 1 or several paracentral scotomas, usually but not always in 1 eye only, without other ocular or visual symptoms. Bilateral cases have been reported in up to 55% of cases. A preceding flu-like illness is the most common reported association, but many cases do not have this association. Rarer reported associations are hormonal contraceptive use, significant coffee consumption, use of epinephrine, hypotensive episodes, and COVID-19 patients. Inquiring about recent vaccination status may also be of benefit.
Slit Lamp Examination
Initially, the anterior segment and funduscopic examinations are usually normal. Central visual acuity remains unaffected. Within 3 days to 2 months after symptom onset, lesions become visible as 1 or more reddish-brown petalloid perifoveal lesions with the tips of the petals pointed toward the fovea.
Signs
Signs of AMN include distinctive reddish-brown wedge-shaped or petalloid lesions on fundus exam. However, in some cases the lesions may be hypopigmented or grayish white. Though rare, lesions may be associated with retinal hemorrhages, macular edema, or optic disc edema.
Symptoms
Patients present with the sudden onset of 1 or more petal-shaped paracentral scotomas, usually involving only 1 eye initially, but both eyes may be affected. There are no other visual or ocular symptoms. The scotomas are relative (not absolute - they interfere with vision in the affected area but do not completely eliminate vision in that area). Scotomas are generally stable over time without changes. Some patients have gradual but incomplete improvement over months, while others may never improve.
All of the patient's scotomas may not appear simultaneously but over days. –
Other symptoms include:
- Scotoma/'shadows'/'spot'
- Mild decreased visual acuity ~ 20/30
- Floaters
- Metamorphopsia
- Photopsia
Clinical Diagnosis
Clinical diagnosis is based on the patient's history and symptoms as described above, generally with only infrared fundus photography and outer retinal changes on SD-OCT seen as described above. Intraretinal hemorrhage may be associated.
Fluorescein angiography, ICG angiography and fundus autofluorescence are often normal. Stratus OCT has been reported to be incapable of detecting this disease, as it has failed to show any retinal abnormalities when performed at the same visit in which a patient's SD-OCT shows the classic abnormalities.
Rare reports of decreased P1 amplitude on multifocal ERG exist, but this test is not performed in the overwhelming majority of case reports, and the mf ERG on this author's patient showed normal P1 amplitudes when performed 17 days after symptom onset.
Diagnostic Procedures
See Diagnosis above. If no lesions are visible on funduscopic examination, infrared fundus photography should show the lesions, and SD-OCT through the lesions should show the aforementioned outer retinal changes. If lesions are visible on funduscopic examination, color fundus photography is also useful for documentation.
- Most important investigations include the near infrared reflectance image and spectral domain OCT
- Near infrared reflectance (Spectralis)- nearly always picks up the lesion. Lesion shape may be wedge, tear-drop, oval, horse-shoe shaped or petalloid.
- Spectral domain OCT (SDOCT)-Outer retina is involved due to involvement of deep capillary plexus. Features include:
- A hypereflective plaque between the outer plexiform and outer nuclear layers.
- Disruption of ellipsoid zone/interdigitation zone
- In late stage, thinning of the outer nuclear layer may be seen
- Fundus photograph may not detect early lesions. Visible lesions are reddish-brown or orange or hypopigmented with tear drop shape pointing towards the fovea.
- Amsler chart- typically shows a corresponding scotoma
- Humphrey/Goldmann visual field may reveal the scotoma
- FFA and ICG angiograms are generally normal
- Autofluorescence- may not detect the lesion or the lesions may be hypo-autofluorescent. Hyper-autofluorescence may be noted in near infrared autofluorescence.
Differential Diagnosis
If a clinic has SD-OCT and infrared imaging capabilities, then acute macular neuroretinopathy should be easily distinguishable from the other items below. However, the differential technically includes:
- Acute Retinal Pigment Epitheliitis (Krill's disease)
- Multiple Evanescent White Dot Syndrome (MEWDS)
- Acute Posterior Multifocal Placoid Pigment Epitheliopathy (APMPPE)
- Central Serous Chorioretinopathy (CSCR)
- Optic Neuritis
- Old inner retinal infarcts
- Paracentral Acute Middle Maculopathy (PAMM)
- AMN should be differentiated from PAMM which presents with similar symptoms of central scotoma, decreased central vision and sometimes, metamorphopsia. Similar to AMN, OCT/OCTA in PAMM shows retinal microvascular alteration; OCT imaging shows hyperreflectivity of the inner nuclear layer (INL), inner plexiform layer (IPL) and sometimes in OPL. No disruption in EZ/IZ is usually detected.
- Several ocular disorders have been associated with PAMM and include retinal vein or artery occlusions, hypertensive and diabetic retinopathies, local retrobulbar anesthesia, pregnancy viral flu-like disease and COVID vaccination.
- The two entities, PAMM and AMN, differ as shown below in the table:
| Paracentral Acute Middle Maculopathy (PAMM) | Acute Macular Neuroretinopathy (AMN) |
| inner retinal involvement | outer retinal involvement |
| hyperreflective plaques in INL and IPL on SDOCT | hyperreflective plaques in OPL and ONL on SDOCT |
| may note thinning of INL with time | thinning of ONL in time |
Management
No medication has shown to improve the condition. Even though there are rare associations with hormonal contraceptive use and excessive coffee consumption, there is no recommendation in the literature that patients should discontinue these if they develop this disease.
Medical Follow-Up
Follow-up is at the discretion and comfort level of the physician. If the diagnosis is considered relatively certain based on history and the presence of classic findings, then follow-up may be every few weeks or months simply to document the course of the disease. No intervention can be performed for this disease.
Prognosis
Some scotomas partially resolve, some do not resolve at all. However, there are no reports of this disease causing meaningful vision loss in an eye. Visual prognosis is usually good.
Suggested Reading
- Turbeville SD, Cowan LD, Gass JD (2003) Acute macular neuroretinopathy: a review of the literature. Surv Ophthalmol 48(1):1–11
- Vance SK, Spaide RF, Freund KB, Wiznia R, Cooney MJ. Outer retinal abnormalities in acute macular neuroretinopathy. Retina. 2011 Mar;31(3):441-5
- Corver HD, Ruys J, Kestelyn-Stevens AM, De Laey J, Leroy BP(2007) Two cases of acute macular neuroretinopathy. Eye 21:1226–1229
- Neuhann IM, Inhoffen W, Koerner S, Bartz-Schmidt KU, Gelisken F. Visualization and follow-up of acute macular neuroretinopathy with the Spectralis HRA+OCT device. Graefes Arch Clin Exp Ophthalmol. 2010 Jul;248(7):1041-4
- Maschi C, Schneider-Lise B, Paoli V, Gastaud P. Acute macular neuroretinopathy: contribution of spectral-domain optical coherence tomography and multifocal ERG. Graefes Arch Clin Exp Ophthalmol. 2010 Nov 20
- Monson BK, Greenberg PB, Greenberg E, Fujimoto JG, Srinivasan VJ, Duker JS (2007) High-speed, ultra-high-resolution optical coherence tomography of acute macular neuroretinopathy. Br J Ophthalmol 91(1):119–120
- Ophthalmology: Expert Consult Premium Edition: Enhanced Online Features and Print (Yanoff, Ophthalmology) by Myron Yanoff MD and Jay S. Duker MD (Dec 11, 2008)
References
- ↑ Bos PJ, Deutman AF. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80(4): 573-84
- ↑ Rennie AT, DeWeerd AJ, Martinez MG, Kay CN. Acute Macular Neuroretinopathy Following COVID-19 mRNA Vaccination. Cureus. 2022 Jul 31;14(7):e27502. doi: 10.7759/cureus.27502. PMID: 36060339; PMCID: PMC9426360
- ↑ 3.0 3.1 Rahimy, E, & Sarraf, D. (2014). Paracentral acute middle maculopathy spectral-domain optical coherence tomography feature of deep capillary ischemia. Curr Opin Ophthalmol, 25(3), 207-212. doi:10.1097/icu.0000000000000045
- ↑ Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET Jr. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538-65.
- ↑ 5.0 5.1 Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH. Acute macular neuroretinopathy and COVID-19 vaccination: Case report and literature review. J Fr Ophtalmol. 2023 Jan;46(1):72-82.
- ↑ Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Casalino G, Arrigo A, Romano F, Munk MR, Bandello F, Parodi MB. Br J Ophthalmol. 2019;103:410–414.
- ↑ Differential diagnosis of retinal vasculitis. El-Asrar AMA, Herbort CP, Tabbara KF. Middle East Afr J Ophthalmol. 2009;16:202–218.
- ↑ Ng XL, Betzler BK, Testi I, Ho SL, Tien M, Ngo WK, Zierhut M, Chee SP, Gupta V, Pavesio CE, de Smet MD, Agrawal R. Ocular Adverse Events After COVID-19 Vaccination. Ocul Immunol Inflamm. 2021 Aug 18;29(6):1216-1224. doi: 10.1080/09273948.2021.1976221. Epub 2021 Sep 24. PMID: 34559576; PMCID: PMC8477588.
- ↑ Protsyk O, Gallego-Pinazo R, Dolz-Marco R. Acute macular neuroretinopathy following Moderna COVID-19 vaccination. J Ophthalmic Inflamm Infect. 2023 Jun 29;13(1):30.
- ↑ Radwan LM, Bou Ghanem GO, Daye GN, Ghazi NG. Acute macular neuroretinopathy associated with intravitreal anti-VEGF injection: A case report. Am J Ophthalmol Case Rep. 2022 Aug 18;28:101687. doi: 10.1016/j.ajoc.2022.101687. PMID: 36046518; PMCID: PMC9421172.
- ↑ David JA, Fivgas GD. Acute macular neuroretinopathy associated with COVID-19 infection. Am J Ophthalmol Case Rep. 2021 Dec;24:101232.
- ↑ Choroidal features of acute macular neuroretinopathy via optical coherence tomography angiography and correlation with serial multimodal imaging. Lee SY, Cheng JL, Gehrs KM, et al. JAMA Ophthalmol. 2017;135:1177–1183
- ↑ Duan J, An J, Li M, Zhang Z, Zhou L, Yin P, Ma J, Shang Q. Topographical Relationship Between Acute Macular Neuroretinopathy and Choroidal Watershed Zone or Patchy Choroidal Filling. Front Med (Lausanne). 2022 Feb 1;9:762609. doi: 10.3389/fmed.2022.762609. PMID: 35178410; PMCID: PMC8843832