Acute Corneal Hydrops
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Acute corneal hydrops (ICD-9 #371.62 & ICD-10 #H18.629)
Disease
Acute corneal hydrops (ACH), an uncommon complication of corneal ectatic disorders, involves sudden-onset corneal edema due to a rupture in Descemet membrane (DM) and can cause impaired vision and eye pain.
Epidemiology
In patients with keratoconus, the incidence of acute corneal hydrops is reported to be between 0.2% and 2.8%.[1][2] It also occurs rarely in keratoglobus and pellucid marginal degeneration.[3][4] Acute corneal hydrops presents most commonly between 20 and 40 years of age.[1][5] Males may have up to double the risk of females to develop ACH.[5][6] The role of family history in its etiology is highly variable.[1][6] In New Zealand, individuals of Pacific Island ethnicity were found to have a higher risk of hydrops compared to individuals of European descent.[6] In the UK, there was a higher prevalence of keratoconus and ACH among South Asian and Black patients.[1]
Risk Factors
- Atopic disease[5]
- Eye rubbing [6]
- Learning disabilities [5]
- Down syndrome[5][7]
- Corneal anatomical variances (such as epithelial thickening, stromal thinning, hyperreflective anomalies, or absence of corneal scarring)[8]
- Steep keratometry[2][5]
- Prior acute corneal hydrops[5]
- Elevated intraocular pressure[7]
Pathophysiology
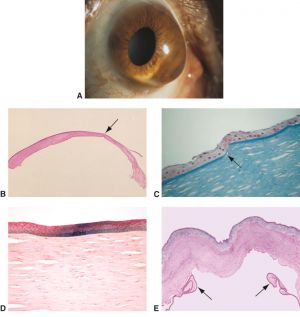
Acute corneal hydrops is believed to result from a break in Descemet membrane and the endothelium, leading to an influx of aqueous humor into the stroma and subsequent formation of corneal edema.[10] Some histopathologic studies provide evidence that disruption of the posterior stroma may contribute to the pathogenesis as well.[11]
Primary Prevention
Effective preventive measures have not been demonstrated for ACH.[12] It is possible that controlling the progression of any underlying corneal ectatic disorders can lower the risk. Minimizing risk factors such as eye rubbing and limiting exposure to allergens in atopic disease may be helpful.
Diagnosis
History

Patients with ACH present with sudden onset decreased vision, photosensitivity, and pain. The episode is often reported to be spontaneous but may be precipitated by coughing, sneezing, nose-blowing, eye-rubbing, strenuous exercise, or other activities that lead to elevated intraocular pressure (IOP).[7] Providers should inquire about a history of keratoconus or other corneal ectasia and risk factors such as atopy and eye rubbing. ACH may be the first sign of keratoconus in many cases.[6]
Physical Examination
Visual acuity is typically decreased. IOP may be artificially low in the setting of corneal edema. During the slit lamp examination of the anterior segment, the degree and extent of corneal edema should be assessed. Corneal edema may limit the view of the posterior cornea, anterior chamber, iris and lens. Seidel testing should be performed; a positive test may indicate transudation of aqueous humor through the corneal stroma rather than corneal perforation. The contralateral eye should be examined for signs of ectasia and predisposing conditions such as vernal or atopic keratoconjunctivitis.
Diagnostic procedures (imaging)
- Anterior Segment Optical Coherence Tomography (AS-OCT): AS-OCT can be used to diagnose ACH as it allows for visualization of the rupture in DM or posterior stroma, and the extent of detachment from the cornea. Additionally, it can be used to monitor the clinical course, including resolution of edema, reattachment of DM, the absence of DM, and scar formation.[15]
 A. AS-OCT of the cornea at 10 days after presentation showing reattachment of DM. Shadowing from the stromal obscures part of the posterior cornea edema. In the visualized portion of the posterior cornea, a water cleft separates a thin layer of posterior stroma and DM from the remaining stroma (arrowheads). B. AS-OCT 5 weeks after presentation demonstrates improving corneal edema with persistent haze, an irregular contour of the central posterior cornea, and protrusions of tissue. The contour of the central posterior cornea is irregular. C. AS-OCT 1 week after ultrathin DSAEK shows a well-attached graft.[14]
A. AS-OCT of the cornea at 10 days after presentation showing reattachment of DM. Shadowing from the stromal obscures part of the posterior cornea edema. In the visualized portion of the posterior cornea, a water cleft separates a thin layer of posterior stroma and DM from the remaining stroma (arrowheads). B. AS-OCT 5 weeks after presentation demonstrates improving corneal edema with persistent haze, an irregular contour of the central posterior cornea, and protrusions of tissue. The contour of the central posterior cornea is irregular. C. AS-OCT 1 week after ultrathin DSAEK shows a well-attached graft.[14] - In-vivo confocal microscopy (IVCM): IVCM, which evaluates tissue at a cellular level, can demonstrate epithelial and stromal edema, which appears as prominent epithelial cell borders and areas of hyper-reflectivity surrounding stromal keratocytes.[16] IVCM can also be used to detect inflammatory cells in the cornea. The presence of these cells longer than 4 weeks is associated with the development of corneal neovascularization.[17]
- Ultrasound biomicroscopy (UBM): UBM is another useful modality for qualitative and quantitative evaluation of corneal hydrops, allowing for assessment of corneal edema and intrastromal clefts, which can be measured and monitored over time. The tear in DM is visualized as the absence of the normal continuous curvilinear hyperintense DM spike. Like AS-OCT, UBM can be used to monitor reapposition of DM, such as after intracameral gas bubble injection.[18]
- Tomography: Corneal tomography provides evaluation of the anterior and posterior corneal curvature and the distribution of corneal thickness. It is routinely used to monitor progression of keratoconus and in evaluation of other corneal ectasias. In patients with acute corneal hydrops and without a known history of ectasia, tomography of the uninvolved eye may be useful for diagnosis.
Differential diagnosis
The differential diagnosis for acute corneal hydrops includes previously undiagnosed or known corneal ectasia including keratoconus (most common), keratoglobus, and pellucid marginal degeneration. One must also consider other causes of corneal edema such as infectious keratitis, uveitis, Fuchs endothelial dystrophy, post-surgical edema, and acute transplant rejection in patients with pertinent positives on history and examination.
Management
Medical therapy
Initial management of ACH is often conservative, as many cases resolve spontaneously within 2 to 4 months. There is a lack of case-control studies investigating topical treatments of ACH and treatment is largely chosen based on anecdotal evidence. Topical measures frequently include an ocular antihypertensive, hypertonic saline, cycloplegia, steroid, and an antibiotic. In the presence of Seidel positivity, which may be related to transudation of aqueous through the edematous cornea instead of perforation, an aqueous suppressant and pressure patching may be utilized.[12][16]
Surgery
- Air/gas: In cases of ACH where the DM is widely separated from the cornea stroma, a pneumatic descemetopexy may be considered to aid in reattachment. Early intracameral injection of air, C3F8 gas, or SF6 gas has been shown to hasten deturgescence of the cornea in ACH. Gas remains in the anterior chamber for a longer duration than air, but requires longer supine positioning with an increased risk of complications. Gases must be used at iso-expansile concentrations.[16] Although both air and gas have shown to decrease the time until edema resolution, there does not appear to be any improvement in final visual acuity or in the need for later corneal transplantation.[12]
- Compression sutures: Like intracameral air and gas, corneal compression sutures may be used to reapproximate the detached DM. Compression sutures may be particularly useful in the presence of intrastromal clefts.[16][19][20] Compression sutures placed perpendicular to the DM tear have been used in isolation or combined with intraoperative air injection.
- Endothelial Keratoplasty: There is recent evidence that endothelial keratoplasty in the acute period after ACH may hasten corneal clearing, improve visual acuity, and lessen the need for later full thickness transplantation. Both Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) have been used to restore normal posterior corneal anatomy following an episode of ACH.[21][22][23] Long-term follow-up is needed to monitor for the formation of corneal scar necessitating more extensive transplantation.
- Penetrating Keratoplasty (PKP)/Deep Anterior Lamellar Keratoplasty (DALK): In cases of resolved ACH that result in a vision debilitating scar, treatment has traditionally consisted of PKP. While PKP has been shown to have excellent success in keratoconus patient, success rates are reduced in those patients following an episode of ACH, particularly if neovascularization develops.[2][16] Given the long-term complications associated with PKP and the relatively young population of ACH patients, DALK may be considered to remove the scar while preserving the patient’s pre-Descemet layer and DM, although it presents technical challenges in this setting.[24]
Complications
The primary complication of ACH is the development of a vision-debilitating scar. See surgical therapy above for management. Other complications include infection, pseudocyst formation and corneal perforation.[12]
Prognosis
The majority of ACH cases resolve spontaneously over the course of 2 to 4 months. As the cornea heals, scar formation can occur. For scars outside the visual axis, this can have an advantageous effect of flattening the cornea and allowing for improved fit of contact lenses. Many times, however, scar formation will impair vision. Prognosis is worse in cases with larger areas of corneal involvement, longer periods of prolonged edema, and neovascularization.[12][25]
Additional Resources
National Keratoconus Foundation https://nkcf.org
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Barsam A, Petrushkin H, Brennan N, et al. Acute corneal hydrops in keratoconus: a national prospective study of incidence and management. Eye . 2015;29(4):469-474.
- ↑ Jump up to: 2.0 2.1 2.2 Tuft SJ, Gregory WM, Buckley RJ. Acute corneal hydrops in keratoconus. Ophthalmology. 1994;101(10):1738-1744.
- ↑ Grewal S, Laibson PR, Cohen EJ, Rapuano CJ. Acute hydrops in the corneal ectasias: associated factors and outcomes. Trans Am Ophthalmol Soc. 1999;97:187-198; discussion 198-203.
- ↑ Sridhar MS, Mahesh S, Bansal AK, Nutheti R, Rao GN. Pellucid marginal corneal degeneration. Ophthalmology. 2004;111(6):1102-1107.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Barsam A, Brennan N, Petrushkin H, et al. Case-control study of risk factors for acute corneal hydrops in keratoconus. Br J Ophthalmol. 2017;101(4):499-502.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Fan Gaskin JC, Good WR, Jordan CA, Patel DV, McGhee CN. The Auckland keratoconus study: identifying predictors of acute corneal hydrops in keratoconus. Clin Exp Optom. 2013;96(2):208-213.
- ↑ Jump up to: 7.0 7.1 7.2 McMonnies CW. Mechanisms for acute corneal hydrops and perforation. Eye Contact Lens. 2014;40(4):257-264.
- ↑ Fuentes E, Sandali O, El Sanharawi M, et al. Anatomic Predictive Factors of Acute Corneal Hydrops in Keratoconus: An Optical Coherence Tomography Study. Ophthalmology. 2015;122(8):1653-1659.
- ↑ © 2021 American Academy of Ophthalmology
- ↑ Stone DL, Kenyon KR, Stark WJ. Ultrastructure of keratoconus with healed hydrops. Am J Ophthalmol. 1976;82(3):450-458.
- ↑ Parker JS, Birbal RS, van Dijk K, Oellerich S, Dapena I, Melles GRJ. Are Descemet Membrane Ruptures the Root Cause of Corneal Hydrops in Keratoconic Eyes? Am J Ophthalmol. 2019;205:147-152.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 Fan Gaskin JC, Patel DV, McGhee CNJ. Acute corneal hydrops in keratoconus - new perspectives. Am J Ophthalmol. 2014;157(5):921-928.
- ↑ © 2021 American Academy of Ophthalmology
- ↑ Blitzer AL, Liles CA, Harocopos GJ, Reidy JJ, Farooq AV. Severe Corneal Hydrops With Suspected Posterior Stromal Rupture Managed With Ultrathin Descemet-Stripping Automated Endothelial Keratoplasty. Cornea. 2021 Apr;40(4):513-515. doi: 10.1097/ICO.0000000000002464.
- ↑ Gokul A, Vellara HR, Patel DV. Advanced anterior segment imaging in keratoconus: a review. Clin Experiment Ophthalmol. 2018;46(2):122-132.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 Sharma N, Maharana PK, Jhanji V, Vajpayee RB. Management of acute corneal hydrops in ectatic corneal disorders. Curr Opin Ophthalmol. 2012;23(4):317-323.
- ↑ Lockington D, Fan Gaskin JC, McGhee CNJ, Patel DV. A prospective study of acute corneal hydrops by in vivo confocal microscopy in a New Zealand population with keratoconus. Br J Ophthalmol. 2014;98(9):1296-1302.
- ↑ Sharma N, Mannan R, Jhanji V, et al. Ultrasound biomicroscopy-guided assessment of acute corneal hydrops. Ophthalmology. 2011;118(11):2166-2171.
- ↑ García-Albisua AM, Davila-Avila N, Hernandez-Quintela E, et al. Visual and Anatomic Results After Sole Full-Thickness Sutures for Acute Corneal Hydrops. Cornea. 2020;39(5):661-665.
- ↑ Yahia Chérif H, Gueudry J, Afriat M, et al. Efficacy and safety of pre-Descemet’s membrane sutures for the management of acute corneal hydrops in keratoconus. Br J Ophthalmol. 2015;99(6):773-777.
- ↑ Tu EY. Descemet Membrane Endothelial Keratoplasty Patch for Persistent Corneal Hydrops. Cornea. 2017;36(12):1559-1561.
- ↑ Palioura S, Chodosh J, Pineda R. A novel approach to the management of a progressive Descemet membrane tear in a patient with keratoglobus and acute hydrops. Cornea. 2013;32(3):355-358.
- ↑ Bachmann B, Händel A, Siebelmann S, Matthaei M, Cursiefen C. Mini-Descemet Membrane Endothelial Keratoplasty for the Early Treatment of Acute Corneal Hydrops in Keratoconus. Cornea. 2019;38(8):1043-1048.
- ↑ Fuest M, Mehta JS. Strategies for Deep Anterior Lamellar Keratoplasty After Hydrops in Keratoconus. Eye Contact Lens. 2018;44(2):69-76.
- ↑ Al Suhaibani AH, Al-Rajhi AA, Al-Motowa S, Wagoner MD. Inverse relationship between age and severity and sequelae of acute corneal hydrops associated with keratoconus. Br J Ophthalmol. 2007;91(7):984-985.

