Acetazolamide Complications in Ophthalmology
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Carbonic anhydrases are a family of zinc-containing metalloenzymes that are found in multiple human tissues and catalyze a reversible reaction of carbon dioxide and water into carbonic acid and bicarbonate ions. These enzymes play an important role in the acid-base homeostasis, pH regulation, and fluid balance of the tissue.[1]
Acetazolamide [(N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)- acetamide] is a nonbacteriostatic sulfonamide derivative and potent carbonic anhydrase inhibitor which has been widely used for the management of glaucoma, idiopathic intracranial hypertension (IIH), cerebrospinal fluid leak, high altitude sickness, and epilepsy.[2]
Current research in acetazolamide therapy includes promising results in low-dose therapy to prevent high-dose methotrexate-incudes toxicities for patients undergoing long-term treatment as in central nervous system lymphoma and acute lymphoblastic leukemia[3] . Other ongoing research demonstrates improvement in obstructive and central sleep apnea in patients on short-term acetazolamide. [4]
In this article, we discuss the adverse events and complications of acetazolamide in the field of Ophthalmology.
Adverse events and Complications
General adverse events
Systemic adverse events have been well documented and reported in the literature. According to the 2014 IIH Treatment Trial patients on acetazolamide were at significantly higher risk of experiencing paresthesia, dysgeusia, gastrointestinal symptoms (nausea, diarrhea, vomiting), and loss of appetite compared to patients belonging to the placebo arm of the trial.[5] Other less common findings were metabolic acidosis, respiratory compromise, hypokalemia, fatigue, polyuria, and calcium phosphate type renal stone formation.[5] In addition, it was shown that the median number of adverse events reported by each patient was five (5) with at least one (1) event reported in 84% of participants.[5]
Sulfonamide antibiotic allergy and cross-reactivity
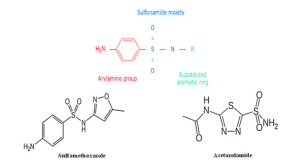
Moreover, there are studies that have suggested that patients with a sulfa allergy should avoid acetazolamide in the light of some severe and life-threatening reactions that could potentially occur as a result of cross-reactivity. In the retrospective study of Lee et al where 34 patients with IIH and self-reported sulfa allergy on acetazolamide (n=13), furosemide (n=7), or both (n=14) were included, it was noted that there is a low likelihood of cross-reactivity between sulfonamide antibiotics and nonantimicrobial sulfonamide medications and this is because of two key structural differences between them which render them less likely to cause allergic reactions.[7] The first key difference is that acetazolamide lacks the arylamine (NH2) side chain at the N4 position which is present in sulfonamide antibiotics and is thought to play a central role in the development of hypersensitivity.[6] The second difference is that it also lacks an aromatic heterocyclic ring and nitrogen group/s at the N1 position.[7](Figure 1) Based on the above findings, the authors concluded that patients with IIH could receive acetazolamide as the risk of experiencing an adverse reaction is low.[7] Furthermore, Strom et al have shown that while there is an association between hypersensitivity reaction after sulfonamide antibiotics and subsequent reaction to nonantimicrobial sulfonamides, this is likely due to susceptibility to allergic reactions rather than to cross reactivity.[8]
Hemopoietic adverse events
Acetazolamide-induced hemopoietic adverse events have been reported in the literature and include pancytopenia, pure thrombocytopenia, aplastic anemia, and agranulocytosis in both adults and pediatric patients.[9] [10] [11] [12] [13] Overall, their incidence is very low and possibly result from immunologic reactions or toxic mechanisms.[13] According to the findings of the IIH Treatment Trial, monitoring of blood cell count is not considered necessary.[5]
Acute Kidney Injury and Electrolyte imbalances
In rare cases, acetazolamide can also induce electrolyte abnormalities such as hypokalemia, metabolic acidosis, and hyponatremia, and thus, its use in patients with decreased renal function is contraindicated as well as in patients with hyperchloremic acidosis.[14] [15] Additionally, due to the natriuretic effect in the proximal tubule and the alkalization of urine, acetazolamide can lead to rhabdomyolysis-induced myoglobinuric renal failure[16], especially when used in patients with ATP shortage and Na+/K+-ATPase dysfunction such as in patients with McArdle disease. [17]Recent case studies have also shown a propensity for acetazolamide intoxication within elderly populations, causing electrolyte and acute kidney injury after routine cataract surgeries that require dialysis.[18][19]
Conversely, in severe cases of hypochloremia or metabolic alkalosis, acetazolamide has been successfully used for the correction of underlying electrolyte abnormalities.[14][20] There are currently no studies that support electrolyte evaluation prior to treatment initiation.
Nephrolithiasis
Acetazolamide has been the pharmacological treatment of choice in patients with IIH due to the safety profile in dosages up to 4 grams per day and for effectively reducing the production of cerebrospinal fluid which can be as much as 50% within 60 to 90 minutes after administration.[21] [22] Moreover, studies have demonstrated that patients on acetazolamide have overall good clinical outcomes with the improvement of papilledema, visual field testing, and a decrease in intracranial pressure.[23] [24] Nevertheless, due to its action on the proximal renal tubules and the alkalization of urine acetazolamide has been considered a risk factor for calcium phosphate stone formation in contrast to loop diuretics such as furosemide which lead to hypercalciuria and thus calcium oxalate stone formation.[25] In 2015, Au et al investigated the effect of daily acetazolamide in patients with IIH and concluded that stone formation is an uncommon side effect and it can most likely take place within the first eighteen (18) months of therapy whereas there are no clinical features that can be associated with its occurrence.[26]
Respiratory Compromise
In the IIH Treatment Trial, two cases of severe respiratory compromises occurred. One patient with a history of asthma and COPD required mechanical ventilation due to multifactorial hypercapneic respiratory failure, whereas the other patient had no previous lung problems or co-morbidities yet developed hyperventilation in response to metabolic acidosis. A case review of these patients concluded that acetazolamide may lead to respiratory complications in patients with or without lung disease, and thus should be monitored for the insidious onset of electrolyte balances and the respiratory compromise.[27]
Myopia
The first case of acetazolamide-induced myopia was reported in 1956 by Marcel Back in a 39-year-old man who was on acetazolamide (250mg/day).[28] Discontinuation of acetazolamide led to symptom resolution whereas a new episode occurred once he started taking the medication again at the same dose. At that point the mechanism of action was considered to be attributed to the changes in salt and water balance while sensitivity factors were thought to contribute to this reaction which results in edema of the ciliary body, lens curvature, and anterior chamber narrowing.[28][29] Since then, a number of case studies have described this phenomenon. One recent case reported significant acetazolamide-induced myopia in a 44-year-old otherwise healthy woman who received a single dose of 125mg for high altitude sickness prophylaxis.[30] Another recent case reported the incidence of acute transient myopia and choroidal detachment following laser capsulotomy and subsequent acetazolamide administration.[31]
The incidence of acetazolamide-induced myopia is not known. The change in myopia usually ranges between 1 to 8 diopters and studies have shown that visual alterations can be experienced as soon as 4 hours after administration or as late as 5 days.[32] Improvement of symptoms is typically noticed within 24 hours after discontinuation but complete resolution can take several days to occur.[33] Dexamethasone is considered an effective alternative to acetazolamide for the prevention of high altitude sickness.[30] [33]
Ciliochoroidal effusions and acute angle-closure glaucoma (ACG)
A number of drugs have been linked to the development of acute angle-closure glaucoma (ACG) in nonglaucomatous patients including acetazolamide, topiramate, anticoagulants, furosemide, and glipizide suggesting an idiosyncratic dose-independent reaction of the uveal part which leads to the development of edema and shallowing of the anterior chamber.[34][35] In these cases discontinuation of medication is required while some authors have recommended administration of systemic and topical steroids, cycloplegics, and aqueous suppressant medications on a case-by-case basis.[34] However, there are no clinical studies supporting their use.
Choroidal effusions in the setting of acetazolamide administration have been well reported in the literature in patients who either undergo ophthalmic procedures or as received prophylactically for high altitude sickness.[30][36] In the majority of cases the patients present with concomitant myopic changes or ACG while in 2017, the first report of choroidal effusions without other ocular manifestations was published by Hari-Kovacs et al.[37]
Conclusions
Acetazolamide has been used for the management of both systemic and ophthalmic conditions. Although uncommon, a number of adverse events and side effects have been associated with its use. Some reactions are mild and reversible, but on rare occasions, its use could lead to irreversible and severe conditions such as anaphylaxis and Stevens-Johnson syndrome.
Since some of the reactions are unanticipated, awareness of these potential complications, early recognition, management, and prevention is crucial for the safety of the patients on acetazolamide. Research is currently ongoing to assess for correlations that may predispose patients to these adverse events with acetazolamide use.
References
- ↑ Merz KM, Hoffmann R, Dewar MJS. The mode of action of carbonic anhydrase. J Am Chem Soc. 2002;111(15):5636-5649. doi:10.1021/JA00197A021
- ↑ van Berkel MA, Elefritz JL. Evaluating off-label uses of acetazolamide. Am J Health Syst Pharm. 2018;75(8):524-531. doi:10.2146/AJHP170279
- ↑ Ku M, Bazargan A, Tam C. Addition of low dose acetazolamide as an adjunct in patients undergoing high dose methotrexate is safe and beneficial. Intern Med J. 2020;50(3):357-362. doi:10.1111/IMJ.14468
- ↑ Schmickl CN, Landry SA, Orr JE, et al. Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis. Chest. 2020;158(6):2632-2645. doi:10.1016/J.CHEST.2020.06.078
- ↑ Jump up to: 5.0 5.1 5.2 5.3 ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott MP. Safety and Tolerability of Acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol. 2016;36(1):13-19. doi:10.1097/WNO.0000000000000322
- ↑ Jump up to: 6.0 6.1 Kelly TE, Hackett PH. Acetazolamide and Sulfonamide Allergy: A Not So Simple Story. https://home.liebertpub.com/ham. 2010;11(4):319-323. doi:10.1089/HAM.2010.1051
- ↑ Jump up to: 7.0 7.1 7.2 Lee AG, Anderson R, Kardon RH, Wall M. Presumed “sulfa allergy” in patients with intracranial hypertension treated with acetazolamide or furosemide: cross-reactivity, myth or reality? Am J Ophthalmol. 2004;138(1):114-118. doi:10.1016/J.AJO.2004.02.019
- ↑ Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349(17):1628-1635. doi:10.1056/NEJMOA022963
- ↑ Hoffman FG, Zimmerman SL, Reese JD. Fatal agranulocytosis associated with acetazolamide. N Engl J Med. 1960;262(5):242-244. doi:10.1056/NEJM196002042620508
- ↑ keisu m, wiholm b ‐e, öst, mortimer. acetazolamide-associated aplastic anaemia. j intern med. 1990;228(6):627-632. doi:10.1111/j.1365-2796.1990.tb00290.x
- ↑ Kodjikian L, Durand B, Burillon C, Rouberol F, Grange JD, Renaudier P. Acetazolamide-induced thrombocytopenia. Arch Ophthalmol. 2004;122(10):1543-1544. doi:10.1001/ARCHOPHT.122.10.1543
- ↑ Maclean R, O’Callaghan U, Lim SH. Acetazolamide-induced severe pancytopenia mimicking myelodysplasia relapse following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;21(3):309-311. doi:10.1038/SJ.BMT.1701077
- ↑ Jump up to: 13.0 13.1 Incecik F, Ozcan N, Ozcanyuz D, Mert G. Acetazolamide-Induced Agranulocytosis in a Patient with Pseudotumor Cerebri. Ann Indian Acad Neurol. 2020;23(5):732. doi:10.4103/AIAN.AIAN_58_19
- ↑ Jump up to: 14.0 14.1 Moviat M, Pickkers P, van der Voort PHJ, van der Hoeven JG. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit Care. 2006;10(1). doi:10.1186/CC3970
- ↑ Carbonic Anhydrase Inhibitors - StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK557736/. Accessed May 22, 2022.
- ↑ Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67(9):272-283
- ↑ Douglas VP, Owji S, Pakravan M, Charoenkijkajorn C, Lee AG. McArdle Disease Rhabdomyolysis Precipitated by Acetazolamide for Idiopathic Intracranial Hypertension. Journal of Neuro-Ophthalmology. 9900. https://journals.lww.com/jneuro-ophthalmology/Fulltext/9900/McArdle_Disease_Rhabdomyolysis_Precipitated_by.44.aspx.
- ↑ Kerber JM, et al. Acetazolamide Intoxication in an Elderly Patient with Diabetes and Chronic Renal Failure after Cataract Surgery. Case Reports in Critical Care 2020: 01 Feb 2020
- ↑ Abou-Mrad RM, Ibrahim M, Osman N, Babu RS. Anuric Acute Kidney Injury Requiring Dialysis Following Acetazolamide Use for Cataract Surgery. Am J Case Rep. 2021 Apr 23;22:e931319. doi: 10.12659/AJCR.931319. PMID: 33888675; PMCID: PMC8077391.
- ↑ Kataoka H. Treatment of hypochloremia with acetazolamide in an advanced heart failure patient and importance of monitoring urinary electrolytes. Journal of Cardiology Cases. 2018;17(3):80-84. doi:10.1016/J.JCCASE.2017.10.003
- ↑ Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25(4):430-436. doi:10.3171/JNS.1966.25.4.0430
- ↑ Wall M, Kupersmith MJ, Kieburtz KD, et al. The Idiopathic Intracranial Hypertension Treatment Trial: Clinical Profile at Baseline. JAMA Neurol. 2014;71(6):693. doi:10.1001/JAMANEUROL.2014.133
- ↑ Çelebisoy N, Gökçay F, Şirin H, Akyürekli Ö. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116(5):322-327. doi:10.1111/J.1600-0404.2007.00905.X
- ↑ Wall M, McDermott MP, Kieburtz KD, et al. Effect of Acetazolamide on Visual Function in Patients With Idiopathic Intracranial Hypertension and Mild Visual Loss: The Idiopathic Intracranial Hypertension Treatment Trial. JAMA. 2014;311(16):1641. doi:10.1001/JAMA.2014.3312
- ↑ Matlaga BR, Shah OD, Assimos DG. Drug-Induced Urinary Calculi. REVIEWS IN UROLOGY. 2003;5(4):227-231.
- ↑ Au JN, Waslo CS, McGwin G, Huisingh C, Tanne E. Acetazolamide-induced nephrolithiasis in idiopathic intracranial hyper tension patients. Journal of Neuro-Ophthalmology. 2016;36(2):126-130. doi:10.1097/WNO.0000000000000330
- ↑ Costello, Fiona MD, FRCPC; Skolnik, Kate MD, FRCPC; Sarna, Justyna MD, PhD, FRCPC; Varughese, Rhea MD, FRCPC. Respiratory Complications Associated With Acetazolamide Use in the Management of Idiopathic Intracranial Hypertension. Journal of Neuro-Ophthalmology: December 2019 - Volume 39 - Issue 4 - p 511-512 doi: 10.1097/WNO.0000000000000813
- ↑ Jump up to: 28.0 28.1 Back M. Transient myopia after use of acetazoleamide (diamox). AMA Arch Ophthalmol. 1956;55(4):546-547. doi:10.1001/ARCHOPHT.1956.00930030550013
- ↑ Muirhead JF, Scheie HG. Transient myopia after acetazolamide. Arch Ophthalmol. 1960;63(2):315-318. doi:10.1001/ARCHOPHT.1960.00950020317015
- ↑ Jump up to: 30.0 30.1 30.2 Rothwell A, Anderson O. Bilateral choroidal effusions after taking acetazolamide for altitude sickness. BMJ Case Reports CP. 2022;15(1):e246145. doi:10.1136/BCR-2021-246145
- ↑ Musetti D, Nicolò M, Bagnis A, Cutolo CA, Traverso CE. Bilateral choroidal detachment and myopic shift after acetazolamide intake for laser capsulotomy. European Journal of Ophthalmology. 2022;32(1):NP51-NP53. doi:10.1177/1120672120974284
- ↑ Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms; Also Systemic Side Effects from Eye Medications. Vol 1. Charles C Thomas Publisher; 1993.
- ↑ Jump up to: 33.0 33.1 Hill AD. Myopic Changes in a Climber after Taking Acetazolamide and the Use of Corrective Lenses to Temporize Symptoms: A Case Report from Mount Kilimanjaro. Wilderness Environ Med. 2016;27(3):397-400. doi:10.1016/J.WEM.2016.04.002
- ↑ Jump up to: 34.0 34.1 Acetazolamide and Bilateral Uveal Effusion With Secondary Acute Angle-Closure Glaucoma - Glaucoma Today. https://glaucomatoday.com/articles/2010-apr/acetazolamide-and-bilateral-uveal-effusion-with-secondary-acute-angle-closure-glaucoma. Accessed May 14, 2022.
- ↑ Pathak-Ray V, Chandran P. Acetazolamide-Associated Idiosyncratic Simultaneous Bilateral Angle Closure and Cross-Sensitivity. Am J Ther. 2020;27(6):E680-E682. doi:10.1097/MJT.0000000000001045
- ↑ Musetti D, Nicolò M, Bagnis A, Cutolo CA, Traverso CE. Bilateral choroidal detachment and myopic shift after acetazolamide intake for laser capsulotomy. Eur J Ophthalmol. 2022;32(1):NP51-NP53. doi:10.1177/1120672120974284
- ↑ András dr HK, Judit dr S, Tamás dr G, et al. Acetazolamid orális alkalmazása mellett jelentkező chorioidealeválás két esete: ismert idioszinkráziás hatás szokatlan megjelenési formája? Orvosi Hetilap. 2017;158(50):1998-2002. doi:10.1556/650.2017.30944

