ADAMTSL4-Related Eye Disorders
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
ADAMTSL4-related eye disorders
Disease
ADAMTSL4-related eye disorders refer to a spectrum of ocular abnormalities caused by mutations in the ADAMTSL4 gene. The most common manifestations of this genetic disorder include isolated ectopia lentis, ectopia lentis et pupillae, congenital cataracts and glaucoma.[1] The condition is commonly bilateral but is often asymmetric.[2]
Ectopia lentis (EL) refers to the displacement of the crystalline lens from its natural position in the eye.[3] This can occur due to a variety of causes, including trauma and and hereditary disorders.[4] Inherited EL is mostly recognized in the context of systemic diseases that feature EL as one of their diagnostic features such as Marfan syndrome (MFS), homocystinuria, Weill-Marchesani syndrome, and others.[4] However, inherited EL can also occur in isolation and in the absence of manifest systemic abnormalities. Conditions that have been reported to lead to isolated EL include autosomal dominant FBN1 (in variants not associated with MFS) and autosomal recessive ADAMTSL4 gene mutations.[4]
Ectopia lentis et pupillae (ELeP) refers to iris abnormalities and displacement of the pupil.[1] The pupils will often appear irregular with an oval or slit shape along with a dilation deficit.[2]
Epidemiology
Overall prevalence of ADAMTSL4 – related disorders is difficult to assess due to limited awareness and research regarding the condition currently. Case studies have been reported in populations across the world.[1] Certain pathogenic variants have been shown to occur at higher prevalence in specific communities and are most likely attributable to founder effects. The most studied variant, a 20-bp deletion (c.767_786del) in exon 6, occurs at higher prevalence in European populations with an estimated homozygous frequency of 1:16,000 in Norway. Based on inferences from shared haplotype lengths, it is thought to have originated in a common European ancestor more than 150 generations or 4000 years ago.[5] In Polynesians, the ADAMTLS4 c.2237G>A variant has an estimated allele frequency of 1:100,000, carrier frequency of 1:132, and homozygous frequency of 1:10,000,000,000.[6] In Bukharian Jews, the ADAMTSL4 c.2663G>A variant has an estimated allele frequency of 1:95, carrier frequency of 1:48, and homozygous frequency of 1:9000.[7] In Amish and Mennonite populations in Ohio, the ADAMTSL4 c.767_786 deletion has an estimated carrier frequency of 1:54.[8][9]
Etiology
The ADAMTSL4 gene resides on chromosome 1q21.2 and contains 17 coding exons.[1] Inheritance of ADAMTSL4 pathogenic variants typically occur in an autosomal recessive fashion.[1] Many previous studies reported homozygous mutations in affected patients, but more recent studies have identified novel compound heterozygous mutations in families with both phenotypes of isolated EL and ELeP.[10][11] [12]More than 13 pathogenic variants have been reported to cause ADAMTSL4-related eye disorder with the 20-bp deletion c.767_786del (p.Gln256ProfsTer38) on exon 6, being most common.[13] These variants introduce premature stop codons via frameshift or transversion mutations, thereby producing a truncated ADAMTSL4 protein end product with a higher risk of anomaly. [14][15] Other known pathogenic variants include missense mutations c.2237G>A (p.Arg746His), c.2594G>A (p.Arg865His), and c.2663G>A (p.Arg888His) that cause critical single amino acid changes in the ADAMTSL4 protein sequence.[1]
Pathophysiology
The A Disintegrin And Metalloproteinase with Thrombospondin motifs (ADAMTS) family includes ADAMTS and ADAMTS-like (ADAMTSL) proteins. ADAMTS proteins are metalloproteases that act as enzymes in multiple biological processes. While ADAMTSL proteins are structurally similar to ADAMTS, they lack the protease domain necessary for enzymatic function.[14] Instead, ADAMTSL proteins are glycoproteins that are secreted into the extracellular matrix (ECM) or remain tethered to the outer cellular membrane.[16] In the ECM, ADAMTS-like 4 (ADAMTSL4) has been found to be associated with fibrillin-1 secretion, assembly, and formation.[17][4][16] Fibrillin-1 is a glycoprotein secreted by fibroblasts that deposits into microfibrils and acts as a major contributor to ECM structure and function.[18] Abnormalities in fibrillin-1 have been found to cause diseases of connective tissues, most notably MFS.
Cell culture experiments have demonstrated that a greater fibrillin-1 deposition area and immunofluorescence signal intensity is achieved in the presence of ADAMTSL4, suggesting that ADAMTSL4 plays a contributory role to the biogenesis and maintenance of fibrillin-1-containing structures.[17] Studies have shown that ADAMTSL4 is expressed in iris and choridal tissues but absent in neurosensory retina.[19] However, the complete function of ADAMTSL4 remains unknown as present findings have yet to account for differences in disease manifestations between ADAMTSL4 and fibrillin-1 defects.
The ciliary zonule of the eye is a ring of extracellular fibers largely composed of fibrillin microfibrils.[20] These zonular fibers anchor to the ciliary bodies on one end and connect to the equatorial region of the crystalline lens on the other, thereby suspending the lens in position within the patellar fossa. The ciliary zonule also serves to transmit ciliary body contraction and relaxation forces to facilitate lens changes in configuration allowing accommodation and focusing of images on the retina.
Dysfunctions of the ciliary zonule and zonular fibers result in instability of the lens. Without adequate structural tensile strength from fibrillin microfibrils, the zonular fibers become unstable and may rupture, leading to lens displacement (ectopia lentis).[1][21] Dislocated lenses can subsequently lose their polarity signaling for cell proliferation and differentiation, and cell dysregulation leads to opacification and congenital or early-onset cataract in some patients.[1][21] Untethered lenses that are not stretched by the zonular apparatus also conform to a more spherical shape known as spherophakia, which results in high myopia. Blurring of the visualized image as a result of a displaced lens triggers axial length elongation, which leads to further refractive error.[13][22] The irregularity of the shape of the lens in this condition also leads to significant astigmatic errors.
The exact pathologic mechanism leading to iris deformities seen in ADAMTSL4-related ELeP remain ill-defined. However, the presence of a persistent pupillary membrane is a common finding, suggesting a component of primary congenital developmental abnormality in the underlying etiology.[23]
Disruption of the fibrillin components contributing to the structure and function of the zonular apparatus is the mechanism by which ADAMTSL4 mutation is believed to lead to ocular symptoms.[17]
Diagnosis
Symptoms
Patients with ADAMTSL4-related eye disorders typically present at a young age with a median age of 3 years old.[24] They are found to be myopic but are otherwise healthy with no medical conditions.[24] However, Family history may or may not be present depending on the genotypes of the parents and siblings.
Physical Examination
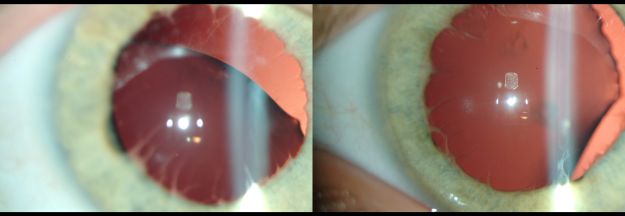
Displacement of the lens and pupil can range from mild to severe, and both components do not need to be present in every eye of every patient.[1] Displacement can occur either unilaterally or asymmetric bilaterally.[1] Other less common phenotypic findings that have been reported in the literature include iris transillumination defects, iridodonesis, microspherophakia, corectopia, coloboma, [25]the frequently present and characteristic persistent pupillary membrane, and early-onset or congenital cataract.[24] Posterior segment findings has be relatively uncommon.
Certain ophthalmic measurements have also been found to be increased in reported cases with ADAMTSL4 mutations, which can be traced back to the underlying pathophysiology of the condition. Mean axial length in a study of 9 individuals with ADAMTSL4-related isolated EL was 27.54 mm (normal range 20-23 mm).[13][26] Elongation of the globe is a known risk factor for retinal detachment, which is also seen at higher frequency in patients with ADAMTSL4 mutation as compared to the general population.[1][22] Increased IOP is also associated with the ADAMTSL4 mutation, but reports of glaucoma in these cases have currently unknown etiologies secondary altered ocular anatomy, post-surgical changes, or other primary processes.[1][22] Studies have also noted possibly slightly increased central corneal thickness (CCT) in affected individuals with ADAMTSL4-related isolated EL at 566.1 µm (normal range 540-560 µm).[13] Increased CCT is known to cause overestimation of applanation tonometry IOP measurement, which also complicates the role of ADAMTSL4 in glaucoma pathology in these cases without evidence of glaucomatous optic nerve degeneration.[1][5]There is also an association with and rare variants of early-onset glaucoma, theorized due to ADAMTSL4 involvement in the anterior segment.[27]
Systemic signs and symptoms are typically absent in cases with ADAMTSL4 mutations, and the ophthalmic abnormalities are usually seen in isolation. However, a few cases of cranial skeletal involvement presenting as craniosynostosis have been reported in patients with ADAMTSL4 mutation that have yet to be widely identified.[17][28]
Diagnostic procedures
ADAMTSL4-related eye disorders can be clinically suspected based on history, symptoms, and detailed ophthalmic examination. Systemic exam is also critical that may point to other potential causes for ectopia lentis. Diagnosis of the condition is based on genetic testing for the presence of pathogenic ADAMTSL4. Various genetic testing strategies can be used based on degree of clinical suspicion and available resources. These include gene-targeted testing (e.g., targeted mutation analysis and single-gene testing) and parallel next-generation sequencing (e.g., multigene panel, whole exome sequencing, whole genome sequencing).[1]
Following identification of the proband, cascade testing of family members and screening of available genetic databases in high-risk communities can also result in diagnosis of additional cases and carriers.
Differential diagnosis and additional tests
Lens dislocation is a prominent characteristic in several hereditary disorders, many of which are more prevalent and better recognized than ADAMTSL4-related eye disorders in the medical and general communities. These include Marfan syndrome, homocystinuria, Weill-Marchesani syndrome, Weill-Marchesani-like syndrome, Traboulsi syndrome, Knobloch syndrome, and Cohen syndrome.[4][13] Rarely, ectopia lentis has also been reported to occur in PAX6 anirida, VSX2 microphthalmia, Loeys-Dietz syndrome, and Sulfite Oxidase Deficiency. [29] Many patients with ADAMTSL4-related eye disorders are often first referred for genetic testing and evaluation for one of these other disorders, most commonly Marfan syndrome and homocystinuria. Genetic testing that includes a panel of all disorders known to feature lens dislocation can help ensure more comprehensive results that lead to the correct diagnosis. There are also some defining features that can help clinicians differentiate ADAMTSL4-related eye disorders from other common inherited diseases with ectopia lentis (Table 1).
Table 1. Summary of prominent findings in ADAMTSL4-related eye disorders, Marfan syndrome, and homocystinuria
| ADAMTSL4-related eye disorders | Marfan syndrome | Homocystinuria | |
|---|---|---|---|
| Average age at diagnosis | 3 years | 19 years | Newborn screening |
| Inheritance | Autosomal recessive | Autosomal dominant | Autosomal recessive |
| Ectopia lentis | + | + | + |
| Pupillary involvement | + | - | - |
| Direction of lens subluxation | Variable | Variable but commonly superotemporal | Inferonasal or anterior |
| Systemic findings | - | + | + |
Comparison with Marfan syndrome
The ADAMTSL4 and fibrillin-1 glycoproteins interact closely in developing tissues, resulting in similar phenotypes from both protein dysfunctions.[16] Both ADAMTSL4-related eye disorders and MFS feature ectopia lentis, increased axial length, refractive errors, early-onset cataract, retinal detachment, and elevated IOP.[1][13][5]
However, a few select features can aid in the differentiation between these two disease processes. Unlike ADAMTSL4, MFS is inherited in an autosomal dominant pattern with 75% of patients having an affected parent.[30] On the other hand, ADAMTSL4 is inherited in an autosomal recessive pattern with parents who are heterozygous carriers and asymptomatic.[1] Additionally, patients with MFS typically present in late childhood or early adulthood with reported median age of diagnosis at 19 years old, whereas patients with ADAMTSL4-related disorder are diagnosed at a significantly younger age with reported medians of 2 or 3 years old.[13][24][31]
Furthermore, MFS has well-established systemic findings, most notably in the cardiovascular and musculoskeletal systems, that are demonstrated in the majority of patients (chest wall deformity in up to 70%, ductal ectasia in more than 90%, scoliosis in more than 50%, and aortic root dilatation in up to 80% of patients).[32][33] On the other hand, ADAMTSL4-related EL occurs mostly in isolation with rare associations with concurrent skeletal abnormalities, specifically craniosynostosis in 3 reported cases.[1][5][17][28] These findings suggest a potential connection between ADAMTSL4 protein contribution to fibrillin microfibrils and TGFβ pathways that affects calvarial sutures that is yet to be elucidated.[28] Nevertheless, the current literature supports that the ophthalmic anomalies in ADAMTSL4-related eye disorders typically occur in the absence of characteristic systemic features.[24]
Comparison with homocystinuria
Another disease that is in the differential diagnosis with MFS and ADAMTSL4-related disease is homocystinuria. Homocystinuria is a rare metabolic condition in which patients have an inherited cystathionine β-synthase (CBS) deficiency, and it is inherited in an autosomal recessive fashion like ADAMTSL4. Homocystinuria also classically features myopia (lenticular) and ectopia lentis in childhood (approximately 1 to 8 years old).[34]
EL in homocystinuria results from ciliary zonule pathology, but major differences in the appearance of the zonule and the lens can be identified on slit lamp examination. In homocystinuria, lack of CBS enzymatic breakdown of homocysteine leads to homocysteine accumulation in plasma and homocysteinylation of fibrillin-1. This disrupts fibrillin-1’s ability to self-aggregate into multimeric microfibrils, inhibiting adequate initial formation of ciliary zonules.[35] On the other hand, patients with ADAMTSL4 mutation have weakened ciliary zonules that exhibit decreased tensile strength. Due to this difference in pathology, patients with homocystinuria have missing zonules or a fringe of short disarranged zonular remnants while patients with ADAMTSL4 mutation have long, stretched zonules and mid-span breakage.[21][36]
The presence of lens dislocation is considered a more severe clinical findings in homocystinuria that is typically seen in patients with a late diagnosis or poorly controlled disease, and bilateral lens dislocation is closely associated with poor biochemical status of disease.[37][38]
Homocystinuria also has systemic findings that typically present during childhood that can aid in differentiation from ADAMTSL4-related eye disorders. Approximately 50% of patients with homocystinuria exhibit developmental delays in childhood, and these are often the first sign of the disease.[2][34] Other neurologic abnormalities are also common with seizures in 21% of untreated individuals, and a higher incidence of psychiatric disorders in adolescents.[34] 50% of affected individuals show signs of osteoporosis by adolescence, and cases of cerebrovascular thromboembolism have been reported in infants.[34]
One feature that clinicians commonly use to differentiate hereditary disorders of lens dislocation is the direction of subluxation. In MFS, the lens is commonly displaced in the superotemporal direction; whereas in homocystinuria, the lens is commonly displaced in the inferonasal direction and anteriorly.[4] The finding of the lens in the anterior chamber is almost pathognomonic of homocystinuria and is extremely rare in MFS. Previous studies have attempted to utilize this feature of displacement direction to differentiate ADAMTSL4-related EL. However, no consistent pattern to lens displacement has been presently identified.[13][39]
Comparison with Loeys-Dietz Syndrome
Loeys-Dietz Syndrome (LDS) and ADAMTSL4-related eye disorders share certain ophthalmic features due to their involvement in connective tissue, but they are distinct entities with differing systemic implications and genetic patterns. Both conditions may present with ocular abnormalities such as increased axial length, refractive errors, and a predisposition to retinal detachment. However, ADAMTSL4-related disorders are particularly characterized by ectopia lentis, which is not a classic feature of LDS.[40]
The genetic inheritance patterns also differ between these two conditions. LDS is typically inherited in an autosomal dominant pattern with a high degree of penetrance, often resulting in multiple affected family members across generations. In contrast, ADAMTSL4-related disorders are inherited in an autosomal recessive pattern, with parents being asymptomatic carriers. This distinction in inheritance has significant implications for genetic counseling and family planning.[41]
Systemically, LDS presents with a wide array of features, most notably in the cardiovascular and skeletal systems, such as aortic aneurysms, arterial tortuosity, craniofacial abnormalities, and scoliosis. These systemic manifestations are integral to the diagnosis of LDS and have profound implications for patient management and prognosis. On the other hand, ADAMTSL4-related eye disorders are generally isolated to the ocular system with minimal or no systemic involvement. While there have been rare associations of craniosynostosis in ADAMTSL4-related cases, the condition primarily affects the eyes without the widespread systemic features seen in LDS.[42]
Management
Treatment and Prognosis
Following diagnosis, a full ophthalmologic examination is warranted in order to determine signs and manifestations of disease. Notably, the exam must include not only slit lamp exam to assess the extent of lens displacement, stretching of zonules, and lens opacification but also dilated fundus exam to rule out any abnormalities in the posterior segment.
Young children with ADAMTSL4-related EL generally do not require surgical intervention and can first be managed with surveillance, a good refraction and the dispension of glasses, and if needed topical anti-glaucoma therapy for elevated IOP.1 There are concerns that the displaced lens may lead to defocusing and deprivation, thus contributing to a higher axial length in EL patients who remain phakic versus those who undergo surgical lens removal. [29] Surgery can be considered in individuals with early-onset visually significant cataracts, severely dislocated lenses, large refractive errors, retinal detachment, glaucomatous damage refractory to medication, and other symptoms refractory to more conservative management or that may increase risk for other ocular pathology.[1][4]
There is currently no standardized surgical management algorithm for patients with ectopia lentis, and careful individualized surgical planning is recommended.[43] When compared to other surgical options, pars plana vitrectomy and lensectomy has been shown to yield the lowest rates of intraoperative and postoperative complications while maintaining stable long-term visual results.[43][44][45]
There are many challenges to IOL placement in the pediatric population, especially in children with ectopia lentis.[46] These include limited anatomical space, capsular elasticity, malleability of eyes still undergoing changes in power and shape, increased reactivity to interventions, and risk of postoperative complications such as visual axis opacification.[45][47] Furthermore, lack of zonular capsular support in ADAMTSL4-related eye conditions prevents IOL placement in the most common and ideal position within the capsular bag. As a result, a variety of extracapsular locations and methods have been used in pediatric cases for IOL implant. IOL can be secured in the anterior chamber or in the posterior chamber with transscleral polyproline or Gore-Tex sutures.[43][44] IOL fixation in the ciliary sulcus is also a possible option but associated with risks of post-operative inflammation and pupillary capture.[45] A more recent approach of intrascleral fixation of the IOL haptics has been used in cases of poor capsular support as a substitute to transscleral suturing.[43] The bag-in-the-lens technique has also been recently reported to significantly lower rates of postoperative visual axis opacification in select cases with potential for utilization in the pediatric population.[47] Many of these techniques are still early in their adoption, so transparent joint decision-making involving the surgeon and patient is indicated. Conservative management for the pediatric population is the most common, leaving the patient aphakic and correcting vision with glasses or contact lenses.[48]
Multiple previous studies have suggested that ophthalmic anomalies of ADAMTSL4-related eye disorders are isolated to the ocular system, and overall prognosis is excellent with no increased risk of systemic disease or effect on lifespan.[13][7] While rare skeletal findings have been identified in some case reports, their association with ADAMTSL4 pathoetiology is unclear.[2][17] Visual prognosis is variable and depends on individual disease manifestations, and development of complications. In a study of 41 patients with ADAMTSL4-related eye disorders who underwent lensectomy, the most significant postoperative complications have included retinal detachment (15%), elevated IOP (10%), and aphakic glaucoma (2%).[24]
Summary
ADAMTSL4-related eye disorders are rare recessively inherited ophthalmic diseases. Early diagnosis can lead to improved patient outcomes with optimal interventions and adequate monitoring for potential complications. At the time of diagnosis, education regarding genetic etiology and counseling of family members is recommended. As ocular abnormalities in ADAMTSL4-related eye disorders typically occur in isolation without characteristic systemic features, overall prognosis for health is excellent. Surgical intervention may become indicated for certain cases, and the need for individualized surgical planning is emphasized due to challenges presented by the population affected and pathology that influences the applicability of surgical techniques. As awareness of the disorder grows, more information regarding the epidemiology, pathology, management, and patient outcomes will become available to researchers, clinicians, and the general public.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 Rødahl E, Elisabeth A, Mellgren C. ADAMTSL4- Related Eye Disorders Summary Genetic counseling GeneReview Scope. 2022;(2):1-14.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Nelson. Ectopia lentis. Am J Ophthalmol. 1946;29(3):735-737.
- ↑ Stellwag. No Title. Published online 1904.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Chandra A, Aragon-Martin JA, Hughes K, et al. A genotype-phenotype comparison of ADAMTSL4 and FBN1 in isolated ectopia lentis. Invest Ophthalmol Vis Sci. 2012;53(8):4889-4896. doi:10.1167/iovs.12-9874
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Christensen AE, Fiskerstrand T, Knappskog PM, Boman H, Roødah E. A novel ADAMTSL4 mutation in autosomal recessive ectopia lentis et pupillae. Invest Ophthalmol Vis Sci. 2010;51(12):6369-6373. doi:10.1167/iovs.10-5597
- ↑ van Bysterveldt KA, al Taie R, Ikink W, Oliver VF, Vincent AL. ADAMTSL4 assessment in ectopia lentis reveals a recurrent founder mutation in Polynesians. Ophthalmic Genet. 2017;38(6):537-543. doi:10.1080/13816810.2017.1309552
- ↑ Jump up to: 7.0 7.1 Reinstein E, Smirin-Yosef P, Lagovsky I, et al. A founder mutation in ADAMTSL4 causes early-onset bilateral ectopia lentis among Jews of Bukharian origin. Mol Genet Metab. 2016;117(1):38-41. doi:10.1016/j.ymgme.2015.11.011
- ↑ Kuang G, Xin B, Sency V, Traboulsi EI, Cruz V, Wang H. Ectopia lentis associated with a 20-base deletion in the ADAMTSL4 gene in the Old Order Amish population. Ophthalmic Genet. 2024 Dec;45(6):602-607. doi: 10.1080/13816810.2024.2406850. Epub 2024 Oct 3. PMID: 39360343.
- ↑ Chiang T, Kloosterboer A, Örge F, Sobol W, Echegaray JJ. Autosomal Recessive ADAMTSL4-Related Isolated Ectopia Lentis in the Ohio Old Order Amish and Mennonite Communities. J Vitreoretin Dis. 2024 Apr 29;8(4):442-451. doi: 10.1177/24741264241249024. PMID: 39148561; PMCID: PMC11323518.
- ↑ Zhao J, Zhou Y, Zhang J, Zhang K, Shang L, Li J. Correlation between novel compound heterozygous ADAMTSL4 variants and primary phenotypes of ectopia lentis et pupillae. 2022;224(November):1-16.
- ↑ Zhou XM, Wang Y, Zhao L, et al. Novel compound heterozygous mutations identified in ADAMTSL4 gene in a Chinese family with isolated ectopia lentis. Acta Ophthalmol. 2015;93(1):e91-e92. doi:10.1111/aos.12399
- ↑ Wei H, Meng X, Qin H, Li X. A novel ADAMTSL4 compound heterozygous mutation in isolated ectopia lentis: a case report and review of the literature. J Med Case Rep. 2023 Dec 26;17(1):532. doi: 10.1186/s13256-023-04272-7. PMID: 38146062; PMCID: PMC10750424.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 Chandra A, Aragon-Martin JA, Hughes K, et al. A genotype-phenotype comparison of ADAMTSL4 and FBN1 in isolated ectopia lentis. Invest Ophthalmol Vis Sci. 2012;53(8):4889-4896. doi:10.1167/iovs.12-9874
- ↑ Jump up to: 14.0 14.1 Ahram D, Sato TS, Kohilan A, et al. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet. 2008;84(2):274-278. doi:10.1016/j.ajhg.2009.01.007
- ↑ Chen ZX, Jia WN, Sun Y, Chen TH, Zhao ZN, Lan LN, Liu Y, Song LH, Jiang YX. Biallelic ADAMTSL4 variants in a Chinese cohort of congenital ectopia lentis: Implications for genotype-phenotype relationships. Hum Mutat. 2022 Dec;43(12):2141-2152. doi: 10.1002/humu.24483. Epub 2022 Oct 17. PMID: 36208099.
- ↑ Jump up to: 16.0 16.1 16.2 Hubmacher D, Apte SS. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biology. 2015;47:34-43. doi:10.1016/j.matbio.2015.05.004
- ↑ Jump up to: 17.0 17.1 17.2 17.3 17.4 17.5 Gabriel LAR, Wang LW, Bader H, et al. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest Ophthalmol Vis Sci. 2012;53(1):461-469. doi:10.1167/iovs.10-5955
- ↑ Asano K, Cantalupo A, Sedes L, Ramirez F. The Multiple Functions of Fibrillin-1 Microfibrils in Organismal Physiology. Organismal Physiology Int J Mol Sci. 2022;2022:1892. doi:10.3390/ijms23031892
- ↑ Chandra A, Jones M, Cottrill P, Eastlake K, Limb GA, Charteris DG. Gene expression and protein distribution of ADAMTSL-4 in human iris, choroid and retina. Br J Ophthalmol. 2013;97(9):1208-1212. doi:10.1136/bjophthalmol-2013-303353
- ↑ Bassnett S. Zinn’s Zonule. doi:10.1016/j.preteyeres.2020.100902
- ↑ Jump up to: 21.0 21.1 21.2 Jones W, Rodriguez J, Bassnett S. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. DMM Disease Models and Mechanisms. 2019;12(1). doi:10.1242/dmm.037283
- ↑ Jump up to: 22.0 22.1 22.2 Neuhann TM, Stegerer A, Riess A, et al. ADAMTSL4-associated isolated ectopia lentis: Further patients, novel mutations and a detailed phenotype description. Am J Med Genet A. 2015;167(10):2376-2381. doi:10.1002/ajmg.a.37157
- ↑ Goldberg MF. Clinical manifestations of ectopia lentis et pupillae in 16 patients. Trans Am Ophthalmol Soc. 1988;86:158-77. PMID: 2979048; PMCID: PMC1298806.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 24.4 24.5 Knight LSW, Mullany S, Taranath DA, et al. The phenotypic spectrum of ADAMTSL4- associated ectopia lentis : Additional cases, complications , and review of literature. 2022;(January):257-268.
- ↑ Chen T, Liu Y, Zhao Y, Wang M, Song L, Jiang Y. Mutations in ADAMTSL4 cause a unique form of autosomal-recessive congenital ectopia lentis. Exp Eye Res. 2024 Nov;248:110090. doi: 10.1016/j.exer.2024.110090. Epub 2024 Sep 13. PMID: 39278391.
- ↑ Bach A, Villegas VM, Gold AS, Shi W, Murray TG. Axial length development in children. Int J Ophthalmol. 2019;12(5):815-819. Published 2019 May 18.
- ↑ Tevar A, Aroca-Aguilar JD, Bonet-Fernández JM, Atienzar-Aroca R, Campos-Mollo E, Méndez-Hernández C, Morales-Fernández L, Leal Palmer I, Coca-Prados M, Martinez-de-la-Casa JM, Garcia-Feijoo J, Escribano J. The Increased Burden of Rare Variants in Four Matrix Metalloproteinase-Related Genes in Childhood Glaucoma Suggests a Complex Genetic Inheritance of the Disease. Int J Mol Sci. 2024 May 25;25(11):5757. doi: 10.3390/ijms25115757. PMID: 38891949; PMCID: PMC11171635.
- ↑ Jump up to: 28.0 28.1 28.2 Gustafson J, Bjork M, Van Ravenswaaij-Arts CMA, Cunningham ML. Mechanism of Disease: Recessive ADAMTSL4 Mutations and Craniosynostosis with Ectopia Lentis. Published online 2022. doi:10.1155/2022/3239260
- ↑ Jump up to: 29.0 29.1 Guo D, Li S, Xiao X, Jiang Y, Wang Y, Jin G, Wang J, Ouyang J, Jia X, Sun W, Wang P, Zheng D, Zhang Q. Clinical and Genetic Landscape of Ectopia Lentis Based on a Cohort of Patients From 156 Families. Invest Ophthalmol Vis Sci. 2024 Jan 2;65(1):20. doi: 10.1167/iovs.65.1.20. PMID: 38190127; PMCID: PMC10777873.
- ↑ Dietz H. FBN1 -Related Marfan Syndrome Summary. Published online 2022:1-24
- ↑ Groth, K.A., Hove, H., Kyhl, K. et al. Prevalence, incidence, and age at diagnosis in Marfan Syndrome. Orphanet J Rare Dis 10, 153 (2015). https://doi.org/10.1186/s13023-015-0369-8
- ↑ Pollock L, Ridout · Ashley, Teh · James, et al. The Musculoskeletal Manifestations of Marfan Syndrome: Diagnosis, Impact, and Management. Curr Rheumatol Rep. 1926;1:3. doi:10.1007/s11926-021-01045-3
- ↑ Saeyeldin A, Zafar MA, Velasquez CA, et al. Natural history of aortic root aneurysms in Marfan syndrome. Ann Cardiothorac Surg. 2017;6(6):625-632. doi:10.21037/acs.2017.11.10
- ↑ Jump up to: 34.0 34.1 34.2 34.3 Sacharow SJ, Picker JD, Levy HL. Homocystinuria Caused by Cystathionine Beta- Synthase Deficiency Summary Genetic counseling. GeneReviews Seattle (WA): University of Washington, Seattle; 1993-2021. Published online 2017:1-21. https://www.ncbi.nlm.nih.gov/books/NBK1524/pdf/Bookshelf_NBK1524.pdf
- ↑ Hubmacher D, Cirulis JT, Miao M, Keeley FW, Reinhardt DP. Functional consequences of homocysteinylation of the elastic fiber proteins fibrillin-1 and tropoelastin. Journal of Biological Chemistry. 2010;285(2):1188-1198. doi:10.1074/jbc.M109.021246
- ↑ Ramsey MS, Dickson DH. Lens fringe in homocystinuria. British Journal of Ophthalmology. 1975;59(6):338-342. doi:10.1136/bjo.59.6.338
- ↑ Rahman. Symposium Recent advances and challenges in the management of retinoblastoma Globe - saving Treatments. BMC Ophthalmol. 2017;17(1):1. doi:10.4103/ijo.IJO
- ↑ Mulvihill A, O’Keeffe M, Yap S, Naughten E, Howard P, Lanigan B. Ocular axial length in homocystinuria patients with and without ocular changes: Effects of early treatment and biochemical control. Journal of AAPOS. 2004;8(3):254-258. doi:10.1016/j.jaapos.2004.01.010
- ↑ Guo D, Li S, Xiao X, Jiang Y, Wang Y, Jin G, Wang J, Ouyang J, Jia X, Sun W, Wang P, Zheng D, Zhang Q. Clinical and Genetic Landscape of Ectopia Lentis Based on a Cohort of Patients From 156 Families. Invest Ophthalmol Vis Sci. 2024 Jan 2;65(1):20. doi: 10.1167/iovs.65.1.20. PMID: 38190127; PMCID: PMC10777873.
- ↑ Braverman AC, Blinder KJ, Khanna S, Willing M. Ectopia lentis in Loeys-Dietz syndrome type 4. Am J Med Genet A. 2020;182(8):1957-1959. doi:10.1002/ajmg.a.61633
- ↑ Loughborough WW, Minhas KS, Rodrigues JCL, et al. Cardiovascular Manifestations and Complications of Loeys-Dietz Syndrome: CT and MR Imaging Findings. Radiographics. 2018;38(1):275-286. doi:10.1148/rg.2018170120
- ↑ Velchev JD, Van Laer L, Luyckx I, Dietz H, Loeys B. Loeys-Dietz Syndrome. Adv Exp Med Biol. 2021;1348:251-264. doi:10.1007/978-3-030-80614-9_11
- ↑ Jump up to: 43.0 43.1 43.2 43.3 Chandra A, Charteris D. Molecular pathogenesis and management strategies of ectopia lentis. Eye. 2014;28:162-168. doi:10.1038/eye.2013.274
- ↑ Jump up to: 44.0 44.1 Liu X, Zheng T, Zhou X, et al. Clinical Study Comparison between Limbal and Pars Plana Approaches Using Microincision Vitrectomy for Removal of Congenital Cataracts with Primary Intraocular Lens Implantation. Published online 2016. doi:10.1155/2016/8951053
- ↑ Jump up to: 45.0 45.1 45.2 Baradaran-Rafii A, Shirzadeh E, Eslani M, Akbari M. Optical Correction of Aphakia in Children. Vol 9.; 2014.
- ↑ Hsu HY, Edelstein SL, Lind JT. Surgical management of non-traumatic pediatric ectopia lentis: A case series and review of the literature. Saudi Journal of Ophthalmology. 2012;26:315-321. doi:10.1016/j.sjopt.2012.05.001
- ↑ Jump up to: 47.0 47.1 Boonstra NE, Haugen OH. Bag-in-the-lens intraocular lens in paediatric cataract surgery: intraoperative and postoperative outcomes. doi:10.1111/aos.14878
- ↑ Musleh M, Bull A, Linton E, Liu J, Waller S, Hardcastle C, Clayton-Smith J, Sharma V, Black GC, Biswas S, Ashworth JL, Sergouniotis PI. The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis. Genes (Basel). 2023 Mar 25;14(4):791. doi: 10.3390/genes14040791. PMID: 37107549; PMCID: PMC10137664.

