Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Pseudotumor cerebri, also known as idiopathic intracranial hypertension (IIH), is a disorder characterized by increased intracranial pressure (ICP) of unknown cause that predominantly affects obese women of childbearing age. Papilledema is the primary ocular finding and may progressively lead to optic atrophy and blindness if no treatment is provided. Variable treatment options are available, but there are no formal guidelines with regards to therapeutic approach.
Disease
Epidemiology and Risk Factors
Among studies performed in the United States, the incidence of IIH was found to be 0.9 to 1.0 per 100,000 in the general population, increasing to 1.6-3.5 per 100,000 in women and to 7.9-20 per 100,000 in overweight women. The disease incidence is variable throughout the world mainly because its occurrence varies according to the incidence of obesity in the region. IIH may be seen in any gender or age group but has a high predilection for females of childbearing age, especially when coupled with obesity. While males are less frequently affected, constituting less than 10% of adult IIH patients, the affected population also tend to be obese and are more likely to sustain worse visual prognosis compared to their female counterparts. Interestingly, in the prepubertal age group, IIH has no particular predilection for obesity or female gender. IIH also has no particular predilection for race, but race may impact visual prognosis in these patients.
Among other possible risk factors, certain systemic illnesses have been associated with IIH, including obstructive sleep apnea, hypothyroidism, anemia, Addison disease, systemic lupus erythematosus, Behçet's syndrome, polycystic ovary syndrome, coagulation disorders, and uremia. The underlying mechanisms of these associations are not yet fully understood. In addition to systemic illnesses, certain medications have also been associated with IIH, including tetracyclines, vitamin A, lithium, anabolic steroids, oral contraceptive pills, nalidixic acid, and cyclosporine.
It is important to note that among pre-pubertal pediatric patients, there is no predilection towards females and more commonly these patients have normal body mass index[1]. Associated conditions include migraines and Down syndrome.
Pathophysiology
The pathophysiology remains unclear but multiple hypotheses have been suggested.
- Cerebral edema was one of the earliest proposed pathological mechanisms for IIH. However, it was quickly criticized because the elevated ICP was not associated with altered levels of alertness, cognitive impairments, or focal neurologic findings typically seen with cerebral edema. Furthermore, no pathologic signs of cerebral edema were documented in these patients.
- Another proposed mechanism involves stenosis of the distal portion of the transverse venous sinuses. This stenosis can result in cerebral venous hypertension and impaired CSF absorption. Farb et al. demonstrated evidence of bilateral venous sinus stenosis in 93% of IIH patients compared to 7% of controls, and numerous other studies have corroborated these findings. However, it is not clear from current literature whether the venous sinus stenosis is the primary cause of the elevated ICP, secondary to the elevated ICP, or an incidental finding.
- Some suggested that an increase in intraabdominal pressure, secondary to obesity, causes increased cardiac filling pressure which impedes venous return from the brain and subsequently leads to an elevated intracranial venous pressure and IIH. However, this hypothesis is unable to explain its presence among the non-obese population group.
- Other studies have suggested a role for vitamin A in IIH pathogenesis based on elevated serum and CSF vitamin A, retinol, and retinol binding protein levels reported in IIH patients. Although the implications of these findings remain unclear, one theory suggests that excess retinol or retinol binding protein in the CSF interferes with CSF resorption. A major study on vitamin A remains to be done.
- It has also been proposed that there may be microthrombosis in the sagittal sinus, of insufficient size to be seen on neuroimaging studies, which is blocking CSF absorption in the arachnoid granulations. However, against this theory is the absence of hydrocephalus which is usually seen with impaired CSF absorption or overproduction.
Few studies have examined the potential role of sex hormones in IIH and the latter remains to be further investigated.
Diagnosis
Clinical Presentation
Although the presentation of IIH is usually non-specific, the following symptoms are among those usually reported:
- Headache: the most commonly reported symptom. It is usually diffuse, non-specific, and may be associated with vomiting. It may also occur with retro-ocular pain.
- Transient episodes of visual loss (usually lasting seconds): often following changes in posture or Valsalva maneuvers.
- Pulsatile tinnitus: a pulse-synchronous sound classically described as a unilateral "whooshing" sound exacerbated with positional changes. It is considered to be specific for the diagnosis.
- Visual disturbance: typically involves the peripheral visual field with an inferonasal defect, arcuate defect, or severe visual field constriction. Visual acuity is not usually affected and is more a sign of fulminant or advanced disease. Central visual field loss may occur in cases when concomitant macular pathology is present.
- Horizontal diplopia: occurs among patients with associated unilateral or bilateral non-localizing sixth cranial nerve palsy.
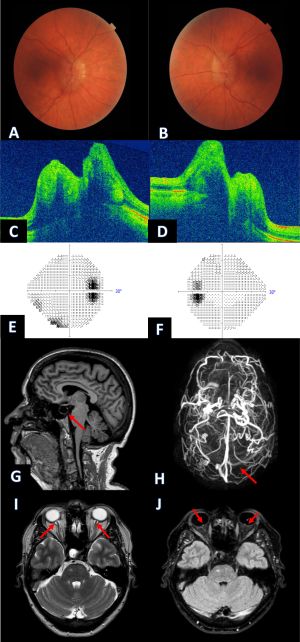
Upon ocular examination, papilledema is the hallmark sign of IIH. It is typically bilateral and symmetric, however unilateral or asymmetrical cases may also occur. The Frisén scale may be used to grade severity of the papilledema. However, the literature describes a few cases of IIH without papilledema among patients presenting with intractable headache and elevated opening pressure on lumbar puncture.
The following may also be present on fundoscopic examination: choroidal compression across macula, choroidal neovascularisation, and retinal elevation around optic nerve head. Finally, 6th cranial nerve palsy may be present as a non-localizing sign of increased intracranial pressure.
Pediatric patients are more likely present with ocular dysmotility[1]. Third and fourth nerve palsies may also be present in pediatric cases.
Diagnostic Criteria
IIH is a diagnosis of exclusion. Nonetheless, diagnostic criteria were established by Dandy in 1937. However, over the years some modifications were provided and the new criteria are now known as the modified Dandy criteria:
- Signs and symptoms of increased ICP (headaches, nausea, vomiting, transient visual obscurations, papilledema)
- No localizing neurologic signs, except for unilateral or bilateral sixth cranial nerve palsy
- CSF opening pressure >25 cm H2O with normal CSF composition
- No evidence of hydrocephalus, mass, structural, or vascular lesion (including venous sinus thrombosis) on imaging
- No other cause of increased ICP identified
Diagnostic Procedures
When evaluating a patient for IIH, a complete ocular examination including a dilated fundus examination, visual field examination, and optic nerve photographs is required. Subsequently, neuroimaging is needed to exclude secondary causes of intracranial hypertension. Magnetic resonance imaging (MRI) and MR venography (MRV) of the brain are usually the imaging modalities of choice. It may show abnormalities suggestive of IIH that are however not specific as they may be seen with other causes of increased ICP. The findings include:
- Flattening of the posterior pole
- Empty/partial empty sella
- Enhancement (with gadolinium) of the prelaminar optic nerve
- Distension of perioptic subarachnoid space
- Vertical tortuosity of the orbital optic nerve
- Intraocular protrusion of the prelaminar optic nerve
- Stenosis of one or both transverse cerebral venous sinuses
Furthermore, a lumbar puncture is recommended for all patients who are suspected of having IIH. The diagnosis is based upon an elevated opening pressure greater than 25 cm H2O taken with the patient lying in the lateral decubitus position. Values between 20 and 25 cm H2O are considered equivocal. In obese or sedated children, pressures up to 28 cm H2O may be considered normal[1]. The CSF must also be studied to rule-out inflammation, tumor cells, and infection. Patients with IIH usually have normal or low protein level, normal glucose levels and a normal cell count.
Differential Diagnosis
IIH is usually a diagnosis of exclusion. The term papilledema usually suggests disc edema/swelling secondary to elevated intracranial pressure, which can have many etiologies in addition to IIH. Therefore, among causes of papilledema the following disease entities must be considered:
- Intracranial mass lesions (tumor, abscess)
- Increased cerebrospinal fluid (CSF) production, eg, choroid plexus papilloma
- Decreased CSF absorption, eg, arachnoid granulation adhesions after bacterial or other infectious meningitis, subarachnoid hemorrhage
- Obstructive hydrocephalus
- Obstruction of venous outflow, eg, venous sinus thrombosis, jugular vein compression, neck surgery
Furthermore, the differential diagnosis of IIH also includes disease entities which look like or lead to unilateral or bilateral disc edema such:
- Pseudopapilledema
- Papillitis
- Hypertensive optic neuropathy
- Central retinal vein occlusion
- Ischemic optic neuropathy
- Infiltration of optic disc
- Leber hereditary optic neuropathy
- Orbital optic nerve tumours
- Diabetic papillopathy
- Thyroid-related optic neuropathy
Management
General Treatment
The goal of treatment is to alleviate symptoms of ICP and preserve vision. Although diagnostic lumbar puncture may provide symptom relief the latter is often transient and requires combination with further long-term therapy.
All obese patients should be encouraged to lose modest amount of weight. Among patients who are obese or overweight, weight loss of about 5-10% has been found to improve symptoms and signs. In a recent study, weight loss allowed a decrease in headaches, papilledema, and ICP. However, the latter option is not effective for acute symptomatic relief and management and therefore needs to be combined with further acute treatment. It is important to ensure preservation of weight loss and avoidance of weight fluctuation in order to minimize the risk of recurrence. Bariatric surgery may be an option among morbidly obese patients. In cases where weight loss alone is insufficient other treatment modalities should be used simultaneously on the long-term.
Medical therapy
Medical therapy is usually considered among patients with mild to moderate disease.
Among the options available, acetazolamide, a carbonic anhydrase inhibitors, is believed to reduce the rate of CSF production and is the first-line medical treatment for IIH. When the latter is inefficient or not tolerated it may be combined with or substituted by:
- Topiramate, a weak carbonic anhydrase inhibitor usually used as an antiepilepsy agent, is considered as a therapeutic option due to its efficacy at improving headaches and allowing weight loss. It has similar efficacy to acetazolamide for visual symptoms.
- Furosemide, a loop diuretic, may also be of use but is not as effective at reducing ICP.
Although steroids were previously routinely recommended in the treatment of IIH, their use is no longer recommended due to their long-term undesired side-effects (mainly weight gain) and the rebound intracranial hypertension caused following withdrawal.
New emerging studies which are of promising future value are evaluating the effect of octreotide, a growth hormone and insulin-like growth factor inhibitor, for the reduction of ICP.
Surgery
Surgical management should be the option of choice among patients with refractory headaches or more severe/ rapidly progressive visual field loss when all other options have failed to prevent progressive visual loss. Corbett and colleagues have provided potential indications for surgery in patients with IIH:
- Development of a new visual-field defect
- Worsening of a previous visual field defect
- Severe visual loss at the time of presentation
- Anticipated hypotension induced by treatment of high blood pressure of renal dialysis
- Psychosocial reasons: non-compliance to medication, inability to perform visual field studies
- Refractory headache
The two most used procedures are CSF diversion via shunt and optic nerve sheath fenestration. The choice of the procedure is based on patients’ signs and symptoms.
Optic nerve sheath fenestration is the preferred surgical procedure for papilledema with associated severe vision loss but no or minimal ICP symptoms (such as headache). It has been shown to preserve or restore vision in 80-90% of patients. The procedure involves incisions in the abnormally bulbous anterior dural covering of the optic nerve sheath which creates an outlet for continuous CSF drainage. Consequently, the CSF no longer distends the sheath and axoplasmic flow in the optic nerve is restored. It is also considered to be the safest approach among patients with renal failure requiring hemodialysis and for vision loss occurring during pregnancy.
CSF shunting produces rapid reduction in ICP and is therefore most beneficial among patients with vision loss and symptoms of raised ICP. Two types are available: lumbo-peritoneal (LP) and ventriculo-peritoneal (VP). Although VP shunting is more difficult, as IIH patients do not have enlarged ventricles, it is the preferred method due to its lower complication rate. Complications of CSF shunting including shunt obstruction, shunt migration, intracranial hypotension, and tonsillar herniation. Sinclair et al. found that shunt revisions were needed in 51% of patients, with 30% requiring multiple revisions.
Venous sinus stenting (VSS) is an emerging procedure based on the findings of venous sinus stenosis in IIH patients. After identification of the stenotic area on MRV, further testing (e.g. digital subtraction venography and manometry) is needed to establish a pressure gradient (typically ≥ 10mmHg) before proceeding with stent placement. VSS has been shown to decrease cerebral venous pressure, leading to increased CSF absorption and consequent decrease ICP. Some studies have demonstrated improvement in symptoms following this procedure. However, despite its possible beneficial outcomes, the procedure may be associated with serious complications such as stent migration, venous sinus perforation, in-stent thrombosis, subdural hemorrhage and recurrent stenosis formation proximal to the stent.
Treatment approach is similar in the pediatric population however surgical intervention should be considered earlier as visual field testing is often inaccurate in this patient population.
Prognosis
The course of the disease is variable and may vary from weeks to years. To date there are no prospective studies that have evaluated the natural history of the disease. Following treatment there is usual improvement and/or disease stabilization. Nonetheless, many patients may not fully recover and demonstrate persistent visual field defect, disc edema or elevated opening pressures on lumbar puncture. Permanent visual loss is the major morbidity and is mostly related to the severity of papilledema. Some studies have identified factors independently associated with a worse visual outcome:
- Gender (male)
- Race (Black)
- Morbid obesity
- Anemia
- Obstructive sleep apnea
- Acute onset of symptoms and signs of raised ICP (fulminant IIH)
Recurrence may occur in 8 to 38% of patients weeks to years following recovery from initial presentation or a prolonged period of stability. Weight gain has been associated with disease recurrence.
Additional Resources
- Boyd K, DeAngelis KD. Idiopathic Intracranial Hypertension. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/idiopathic-intracranial-hypertension-list. Accessed March 13, 2019.
- Patient Information Brochure. North American Neuro-Ophthalmology Society (NANOS). https://www.nanosweb.org/IIH _patient brochure Accessed March 3rd, 2025
References
- Albert D, Jakobiec FA. Principles and practices of ophthalmology. 3e edition. USA. Saunders. 2008.
- Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2012; 83: 488-494.
- Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension.Arch Neurol 1989;46(10):1049.
- Deftereos SN, Panagopoulos G, Georgonikou D, et al. Treatment of idiopathic intracranial hypertension: is there a place for octreotide? Cephalgia 2011. 31: 1679-80.
- Degnan AJ, Levy LM. Pseudotumor Cerebri: brief review of clinical syndrome and imaging findings. Am J Neuroradiol 2011; 32: 1986-93.
- Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418-24.
- Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59(10):1492.
- Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol 2004;24(2):138-45.
- Gans MS. Idiopathic intracranial hypertension. Medscape. http://emedicine.medscape.com/article/1214410-overview. [November 2012].
- Gerstenblith AT, Rabinowitz MP. The Wills Eye Manual:office and emergency room diagnosis and treatment of eye disease. 6th edition. USA. Lippincott Williams & Wilkins. 2012.
- Shah VA, Kardon RH, Lee AG, Corbett JJ, Wall M. Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience.Neurology 2008;70(8):634.
- Taktakishvili O, Shah VA, Shahbaz R, Lee AG. Recurrent idiopathic intracranial hypertension.Ophthalmology 2008;115(1):221.
- Thurthell MJ, Wall M. Idiopathic Intracranial Hypertension (Pseudotumor Cerebri): recognition, treatment, and ongoing management. CurrTreat Options Neurol. Epub Nov 2012.
- Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri). Curr Neurol Neurosci Rep 2008;8(2):87-93.
- Wall M. Idiopathic Intracranial Hypertension. Neurol Clin 2010; 28(3):593 - 617.
- Chen, J. & Wall, M. Epidemiology and Risk Factors for Idiopathic Intracranial Hypertension. "Int. Ophthalmol. Clin." (2014). doi:10.1097/iio.0b013e3182aabf11
- Giridharan, N. et al. Understanding the complex pathophysiology of idiopathic intracranial hypertension and the evolving role of venous sinus stenting: a comprehensive review of the literature. "Neurosurg. Focus" (2018). doi:10.3171/2018.4.focus18100
- Sinclair A., Kuruvath S., Sen D., Nightingale P., Burdon M., Flint G. (2011) Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. "Cephalalgia" 31: 1627–1633.

