Ocular Features of Mucopolysaccharidosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity and Pathophysiology
Mucopolysaccharidoses (MPSs) are a heterogenous and rare group of lysosomal storage disorders. Lysosomes are present in all nucleated cells, and they are part of a complex intracellular recycling system involved in the degradation of large macromolecules. MPSs are caused by inherited defects in lysosomal enzymes resulting in widespread intra- and extra-cellular accumulation of glycosaminoglycans (GAGs). They are characterized by a reduction in the activity of specific lysosomal enzymes involved in the breakdown and catabolism of GAGs, with progressive accumulation within the lysosome.[1] [2] [3]
Classification
The nomenclature for the different types of MPS is historical. The nomenclature, enzyme defect, type of glycosaminoglycan, gene locus and inheritance pattern are distinguished in table 1.
Genetics
All types of MPSs are inherited in an autosomal recessive manner, with the exception of MPS type 2 (Hunter), which is X-linked recessive. MPS, like many other metabolic diseases, present a variable phenotype. In some patients, the presentation may be in utero or in the newborn period, whereas in others, even with the same enzyme deficiency (but probably a different genetic mutation), the onset may be in late adulthood.[2] [3]
Systemic manifestations
Lysosomes are present in all nucleated cells and consequently GAGs may potentially accumulate in every tissue. Like other metabolic disorders, MPSs are progressive and unremitting. The GAGs deposits modify the cellular ultrastructure, leading to subsequent dysfunction. The systemic manifestations include characteristic facial dysmorphism, dental caries and abscesses, growth restriction, skeletal abnormalities (dysostosis multiplex), structural cardiac valvular dysfunction, middle ear disease and hearing loss, upper airway obstruction, recurrent umbilical and inguinal hernia and variable central nervous system manifestations including cognitive impairment. Ocular involvement is frequent and may lead to severe visual impairment. GAGs may accumulate and may involve the sclera, cornea, trabecular meshwork, retina, optic nerve, and posterior visual pathways.[1] [2]
Diagnosis
Ocular features
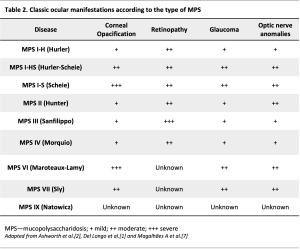
Ocular features in MPSs are very frequent, many of those represent an early manifestation of MPS and result in significant visual impairment. The most common (classic or typical features) include corneal clouding, ocular hypertension and glaucoma, retinopathy and optic disc involvement (optic disc swelling or atrophy).[2] Other features include scleral and choroidal changes,[4] [5] [6] refractive errors, ocular motility disorders, pseudo-exophthalmos, shallow orbits and ptosis.[2] Ocular pathology is common in all types of MPS, but its frequency, severity and ocular localization vary according to the MPS subtype. They are particularly common in MPS types I, VI, and VII. Even among the same subtype, the phenotypic presentation may vary in accordance with genotypic variations, resulting in different enzymatic activity and severity of GAG deposition. Better control of the systemic comorbidities with bone marrow hematopoietic stem cell transplant (HSCT) and enzymatic replacement therapy (ERT) and the increase in life expectancy allowed the identification of new, late-onset ocular manifestations in MPS patients. In addition, new imaging techniques have introduced the possibility of better characterizing and understanding these manifestations.
Corneal clouding/ opacification
Corneal opacification is the most common and classic eye finding of MPS.[7] The optical properties of the cornea reside in its transparency, which in turn depends on its fibrillar and cellular arrangement. The extracellular matrix is uniform, the collagen fibrils are disposed parallelly with the same size and equally spaced, and the keratinocytes are transparent.[8] [9] The intra and extracellular accumulation of GAG lead to keratinocyte degeneration and disorganization of the collagen fibrils. All corneal layers may be compromised, but the Descemet membrane and endothelium are only affected in more advanced stages.[10] [11] [12] The corneal opacification associated with MPS is described as "ground glass".[2] It is more frequent in types I, VI and VII, but it can occur in other types (table 2), with a variable age of onset, with some cases beginning in the first year of life. The prevalence in patients with MPS type I ranges between 80 and 100%.[13] In a tertiary centre in Portugal, all 5 patients with MPS type VI presented corneal clouding and this was the first ocular manifestation.[14] In fact, corneal clouding is the most frequent ocular feature in MPS VI.[3] Initially, it may be asymptomatic or manifested by photophobia. Without treatment, corneal accumulation of GAG is progressive, leading to visual acuity loss. Corneal clouding is diffuse from limbus to limbus[1] and it can interfere with the evaluation of the anterior and posterior segments. Corneal clouding can be graded from I to IV: I - normal cornea; II - mild opacity: iris and fundus details are visible; III - opacity interferes with the vision of iris and fundus details; and IV - anterior chamber and fundus cannot be evaluated.[1]
Other corneal findings
The cornea is naturally avascular. There may be peripheral vascularization as a consequence of chronic corneal edema, due to IOP elevation, or secondarily to corneal exposure associated with exophthalmos.[1][2] Increased corneal opacification, thickness and intraocular pressure (IOP) tend to occur simultaneously.[2][10][12] Some authors have suggested that the deposition of GAG in the corneal stroma causes a progressive increase in corneal thickness.[2][15] In a case series enrolling 5 patients with MPS type VI, during ophthalmologic follow-up, all patients exhibited progressive corneal opacity and thickening, with maximum pachymetry values of 1500 μm, a value almost three times greater than the normal cornea.[14]
Glaucoma and Ocular Hypertension (OHT)
These two conditions have been mainly described in cases of MPS I and VI (table 1).[2][16] [17] The prevalence of glaucoma may vary between 2.1% and 12.5% of the patients with MPS, according to the subtype of MPS.[18] However, the diagnosis of glaucoma and/or OHT is a challenge in patients with MPS. On one hand, OHT may constitute an artifact attributed to thickened and stiffened corneas, not reflecting a real pressure.[14] On the other hand, as described by Magalhães A. et al., optic neuropathy previously attributed to glaucoma may in fact be due to progressive posterior pressure and compression of the fibers in the retro-ocular portion of the optic nerve due to chronic hydrocephaly experienced in patients with MPS VI. This mechanism was recently proposed with the designation of “posterior glaucoma”, a late manifestation observed in MPS VI.[14] The “classical” glaucoma (an intraocular condition) in patients with MPS may have a dual pathophysiology. It can be open angle glaucoma due to the accumulation of GAG in the trabecular meshwork disrupting outflow,[1][2] [16] [17] or angle-closure glaucoma as a consequence of GAG deposition in several ocular tissues, leading to thickening of the peripheral cornea, iris, and ciliary body, and the formation of membrane-like structures on the surface of the iris or iridociliary cysts. The HTO can also be secondary to corneal keratoplasty. Therefore, IOP measurement and gonioscopy are of extreme importance, when possible. Ultrasound biomicroscopy or optical coherence tomography of the anterior segment are ancillary exams to consider. To measure IOP, Icare tonometry seems to provide values higher than those of applanation tonometry (Goldman, Perkins and Tonopen).[18] Care should be taken when interpreting high IOP values in corneas with particularly high central thickness (CCT) measurements and corneal hysteresis.[10][12][18][19] However, no correlation has been established yet between the degree of corneal opacification and CCT or IOP.[18] The evaluation of the optic disc is sometimes impossible due to corneal opacification, and the analysis of the visual fields may be biased by concomitant retinopathy (MPS I and II), apart from the possibility of lack of collaboration. Moreover, patients with MPS may also present optic disc edema or atrophy, impairing even further the clinical evaluation of the neuroretinal rim and interpretation of the visual fields. Therefore, a clear diagnosis of OHT and glaucoma based on structural and functional evaluation is difficult. Other examinations should be considered for monitoring (IOP, visual acuity, corneal diameter, axial length, and eventually electrophysiological examinations).[7] It is important to remember MPS in the differential diagnosis of congenital glaucoma, when evaluating a child with corneal opacification and high IOP.[7]
Retinopathy
GAG deposition in the retinal pigment epithelium and in the photoreceptor matrix lead to the progressive loss of these cells[1][20] MPS-associated pigmentary retinopathy progresses very insidiously and is usually a late manifestation. The age of onset depends on the severity of the phenotype.[1] The severity of the retinopathy is variable in all subtypes of MPS I and MPS II, while it is frequently moderate to severe in MPS III (Sanfilippo).[2][21] Pigmentary retinopathy can also occur in MPS IV (Morquio) (table 1).[2][22] Night blindness and peripheral vision loss are the typical manifestations, while impairment of central vision occurs in the most advanced stages. Fundus examination may show signs of RPE atrophy and pigment clumps; at an advanced stage, optic disc pallor and indistinct foveal reflex can occur. Fundoscopy can be challenging as visualization can be often masked by the corneal findings.[22] New imaging techniques have provided new perspectives regarding MPS. Recently, several new findings were described in a patient with MPS II, with mid-periphery retinal pigmentary changes, with a macular-sparing annular atrophy of the retinal pigment epithelium, and focal pigment clumping. Spectral domain optical coherence tomography using enhanced depth imaging (EDI SD-OCT) revealed a highly irregular choroidal-scleral interface, a bilateral symmetric parafoveal atrophy of the photoreceptor layer, with a prominent central external limiting membrane (ELM) and a circumferential transition zone where the ELM was still present but the ellipsoid zone (EZ) band was undetectable. Fundus autofluorescence revealed a bilateral symmetric hyper-autofluorescent parafoveal ring, with precise anatomic correspondence to the transition zone (TZ) described in the OCT. A mottled hyper/hypo autofluorescent pattern was present in the mid-periphery of the retina. It was also described for the first time the presence of drusen on the optic disc in this disease.[23] A recent case report [5] described the SD-OCT and infrared and en face OCT retinal findings in a patient with MPS I (Hurler's syndrome). Although having initiated early treatment with HSCT and ERT and the fundus examination was clinically normal, the SD-OCT assessment revealed in the foveal area, in both eyes, increased thickness of the hyperreflective band of the ELM. The other outer retinal bands (the myoid, ellipsoid, and interdigitation zones, the RPE, and Bruch's membrane) were within normal limits. In the parafoveal and perifoveal regions, SD-OCT displayed a loss of the interdigitation, ellipsoid, and myoid zones, loss of the ELM, and thinning and eventual loss of the outer nuclear layer (ONL). The scans, close to the fovea, also revealed small cysts in the ONL, inner nuclear layer (INL), and ganglion cell layer (GCL). Fundus infrared imaging revealed a bilateral hyperreflective ring centered on the fovea that was more evident in the left eye. It also revealed hyporeflective areas in the temporal extrafoveal regions in both eyes. Besides the fundoscopic and imaging findings in MPS-associated retinopathy, an abnormal electroretinogram (ERG) can be observed, with initial impairment of the rod-mediated responses, preceding cone abnormalities.[2][24]
Retinal folds
Retinal folds form a rare manifestation. Delleman and de Jong[25] described a case of MPS II with parafoveal retinal folds and changes in the peripheral EPR.
Optic disc involvement
Optic disc swelling and subsequent optic nerve atrophy may occur in all types of MPS, but it is much more frequent in patients with MPS I and MPS VI,[2] [3] with approximately half of them presenting swollen optic discs. The prevalence of optic nerve atrophy in these patients ranges between 8 and 19%. Optic Disc swelling can result from thickening of the sclera due to GAG deposition, which may cause an impingement of the optic nerve at the level of the lamina cribrosa. Alternatively, papilledema can arise secondary to increased intracranial pressure (ICP).[21] Optic nerve atrophy can occur by several mechanisms:
- Increased IOP and glaucoma
- Accumulation of GAG within ganglion cells and secondary degeneration
- Impingement of the optic nerve head
- Communicating hydrocephalus with increased ICP
In a recent case series,[14] five of 12 patients with MPS VI presented hydrocephalus, progressive enlargement of the subarachnoid space and optic nerve atrophy. Hydrocephalus is an early manifestation of MPS, which is known to occur in types I, II, III, VI and VII, and is usually slowly progressive. Its exact pathophysiology in MPS has not been stablished yet, but it is probable due to a complex interplay between changes in the cerebrospinal fluid dynamics (CSF) and inflammatory disease.[26] The enlargement of the third and lateral ventricles are characteristic imaging findings in cases of raised ICP.[27]
Other ocular features
Choroid and Sclera
The thickening of the sclera has been recently better characterized by SD-OCT EDI. Magalhães A. et al. described a patient with MPS VI (Maroteaux-Lamy Syndrome) who presented with thickening of the sclera and thinning of the underlying choroid, documented by EDI SD-OCT, in which it was hypothesized that this could be due to GAG deposits in the sclera.[6] In the same tertiary center, a case of MPS IV (Morquio S.) was recently published using EDI SD-OCT with demonstration of increased thickness of the subfoveal choroid (and not thinning) sparing the retina, without simultaneous GAG deposition in the RPE or retina. [4]
Ocular motility and refraction errors
Ocular motility disorders, especially exotropia, are very common in patients with MPS.[1][21][28] In the case series of Ashworth et al., 37% of patients with MPS I (IH) and 25% with MPS VI manifested a strabismus.[21] Refractive problems, especially hyperopia, are also common in MPS patients. Hyperopia occurs in more than 90% of patients with MPS I (all subtypes) and with MPS VI.[21] [28][29] It may be due to the increased stiffness of the cornea and sclera, a more flattened corneal curvature, and a reduction in the refractive power due to accumulation of GAG or by a shortening of the length axial due to thickening of the sclera.[28] [30] [31]
Management
Previously, the ocular management of many patients was conservative due to their short lifespan and intellectual impairment. However, treatments such as hematopoietic stem cell transplantion (HSCT) and enzyme replacement therapy (ERT) are leading to a longer and better quality of life for many MPS patients. The associated ocular problems are often difficult to manage and there are many anesthetic, surgical and medical considerations. Management of the classic ocular features are described.
Corneal opacification
Despite systemic treatment (HSCT and ERT), no current treatment halts the progression of corneal clouding. Keratoplasty can be a beneficial intervention for patients with corneal opacification and should be considered if vision is impaired or view of the fundus precludes sufficient evaluation of the optic nerve and retina.[32] Without systemic treatment, the graft may become opacified due to GAG deposition in donor keratocytes, which can occur as early as within one year after corneal transplantation.[33] The efficacy of transplantation depends on the type of GAG and severity of the opacification. Penetrating keratoplasty (PK) is the most performed technique, with variable success rates that can be as high as 94%.[32] However, the success rates have been often limited to case series. Since endothelial function is preserved until a more advanced stage, deep anterior laminar procedures (DALK) can be an option, with a smaller risk of rejection but still with good visual outcomes.[10][32][34]
OHT and Glaucoma
Topical treatment is usually the first line treatment to lower IOP, and usually more than one drug is needed. Laser iridotomy is performed in cases of angle closure (when corneal transparency permits). Nevertheless, surgical approach is frequently required, including trabeculectomy ± mitomycin C, ciliary ablation and sometimes a tube shunt.[7]
Retinopathy
There is no current treatment for this late manifestation. However, a better understanding of the role played by the neural structures in the retina, along with new transplant techniques of stem cells, provide a renewed hope in the treatment of retinopathy.[7]
Optic neuropathy
The gold standard for treatment of communicating hydrocephalus is the placement of a ventriculoperitoneal shunt (VPS). Given the deleterious effect of hydrocephalus, a timely and effective treatment is indicated. Aliabadi et al.[35] developed an algorithm to evaluate the need for a shunt in patients with MPS before HSCT; indications for referral include elevated ICP, clinical signs of hydrocephalus or combined clinical deterioration with increased ventricular size on imaging scans. Another procedure, the endoscopic third ventriculostomy (ETV) with or without choroid plexus coagulation (CPC), was proposed as a possible alternative method.[26][36] ETV is a neuroendoscopic procedure involving creation of an opening in the floor of the third ventricle that bypasses CSF flow obstruction, allowing the CSF to flow directly to the basal cisterns. This technique might be considered in cases with an obstructive component. There is only one publication in which this intervention was successfully performed in a patient with MPS VI presenting symptomatic hydrocephalus resistant to conservative treatment.[36]
Prognosis
There is a wide spectrum of clinical phenotypes, ranging from those disorders that are fatal in the first months of life to those compatible with a normal lifespan. Current ERT and HSCT provide a better quality of life and longer life expectancy for many MPS patients.
Conclusion
The ophthalmologist plays an important role in the early diagnosis and follow-up of MPS. Systemic therapies have increased the life expectancy of these patients. The advancements of new technological tools for detection of early and new ocular manifestations are shifting the paradigm from a perspective of expanding the lifespan of this patients to improving secondary outcomes like vision. With MPS patients reaching a longer lifespan, the quality of life and its adequate assessment are of utmost importance. Health-related quality of life tools that utilize patient reported outcomes to address patient parameters such as symptoms, functioning (activity and limitations), or quality of life, have been used to supplement traditional biomedical endpoints.[37] Ultimately, visual status assessment, including patient and guardian interview, examination and visual function questionnaires [38] constitute other matter of concern in this technological modern era where sight is considered the most valued sense.[39] The ophthalmologist therefore has an important role as part of the multidisciplinary management of these patients, whose visual problems may be additional to multiple other physical and intellectual impairments.
Additional Resources
- Mucopolysaccharidoses. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/mucopolysaccharidoses Accessed June 15, 2023.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Del Longo, A., E. Piozzi, and F. Schweizer, Ocular features in mucopolysaccharidosis: diagnosis and treatment. Ital J Pediatr, 2018. 44(Suppl 2): p. 125.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Ashworth, J.L., et al., Mucopolysaccharidoses and the eye. Surv Ophthalmol, 2006. 51(1): p. 1-17.
- ↑ 3.0 3.1 3.2 3.3 Tomatsu, S., S. Pitz, and U. Hampel, Ophthalmological Findings in Mucopolysaccharidoses. J Clin Med, 2019. 8(9).
- ↑ 4.0 4.1 Magalhaes, A., et al., Increased Choroidal Thickness in Morquio Syndrome. Case Rep Ophthalmol, 2021. 12(3): p. 816-823.
- ↑ 5.0 5.1 Magalhaes, A., et al., Macular Changes in a Mucopolysaccharidosis Type I Patient with Earlier Systemic Therapies. Case Rep Ophthalmol Med, 2021. 2021: p. 8866837.
- ↑ 6.0 6.1 Magalhaes, A., et al., Fundoscopic Changes in Maroteaux-Lamy Syndrome. Case Rep Ophthalmol Med, 2019. 2019: p. 4692859.
- ↑ 7.0 7.1 7.2 7.3 7.4 Magalhães, A., et al., Aproximación quirúrgica al paciente con mucopolisacaridosis - Tratamiento quirúrgico de las complicaciones oftalmológicas en los pacientes con mucopolisacaridosis. (awaiting publication).
- ↑ Rada, J.A., P.K. Cornuet, and J.R. Hassell, Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res, 1993. 56(6): p. 635-48.
- ↑ Jester, J.V., et al., Postnatal corneal transparency, keratocyte cell cycle exit and expression of ALDH1A1. Invest Ophthalmol Vis Sci, 2007. 48(9): p. 4061-9.
- ↑ 10.0 10.1 10.2 10.3 Bruscolini, A., et al., Involvement of the Anterior Segment of the Eye in Patients with Mucopolysaccharidoses: A Review of Reported Cases and Updates on the Latest Diagnostic Instrumentation. Semin Ophthalmol, 2017. 32(6): p. 707-714.
- ↑ Morris, E., et al., Corneal endothelial specular microscopy following deep lamellar keratoplasty with lyophilised tissue. Eye (Lond), 1998. 12 ( Pt 4): p. 619-22.
- ↑ 12.0 12.1 12.2 Fahnehjelm, K.T., E. Chen, and J. Winiarski, Corneal hysteresis in mucopolysaccharidosis I and VI. Acta Ophthalmol, 2012. 90(5): p. 445-8.
- ↑ Pastores, G.M., et al., The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab, 2007. 91(1): p. 37-47.
- ↑ 14.0 14.1 14.2 14.3 14.4 Magalhaes, A., et al., Visual impairment in mucopolysaccharidosis VI. JIMD Reports, 2022(1- 9).
- ↑ Alroy, J., M. Haskins, and D.E. Birk, Altered corneal stromal matrix organization is associated with mucopolysaccharidosis I, III and VI. Exp Eye Res, 1999. 68(5): p. 523-30.
- ↑ 16.0 16.1 Nowaczyk, M.J., J.T. Clarke, and J.D. Morin, Glaucoma as an early complication of Hurler's disease. Arch Dis Child, 1988. 63(9): p. 1091-3.
- ↑ 17.0 17.1 Cantor, L.B., J.A. Disseler, and F.M. Wilson, 2nd, Glaucoma in the Maroteaux-Lamy syndrome. Am J Ophthalmol, 1989. 108(4): p. 426-30.
- ↑ 18.0 18.1 18.2 18.3 Ashworth, J., et al., Assessment and diagnosis of suspected glaucoma in patients with mucopolysaccharidosis. Acta Ophthalmol, 2015. 93(2): p. e111-7.
- ↑ Connell, P., et al., Central corneal thickness and its relationship to intraocular pressure in mucopolysaccararidoses-1 following bone marrow transplantation. J AAPOS, 2008. 12(1): p. 7-10.
- ↑ Mack, H.G., R.C.A. Symons, and G. de Jong, Bull's eye maculopathy and subfoveal deposition in two mucopolysaccharidosis type I patients on long-term enzyme replacement therapy. Am J Ophthalmol Case Rep, 2018. 9: p. 1-6.
- ↑ 21.0 21.1 21.2 21.3 21.4 Ashworth, J.L., et al., The ocular features of the mucopolysaccharidoses. Eye (Lond), 2006. 20(5): p. 553-63.
- ↑ 22.0 22.1 Kasmann-Kellner, B., et al., Ocular changes in mucopolysaccharidosis IV A (Morquio A syndrome) and long-term results of perforating keratoplasty. Ophthalmologica, 1999. 213(3): p. 200-5.
- ↑ Penas SC, Magalhães A, Breda JR, Cruz JM, Brandão EM, et al. Fundus Autofluorescence and Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography in Hunter Syndrome-New Insights. Journal of Clinical & Experimental Ophthalmology 7, doi:10.4172/2155-9570.1000577 (2016).
- ↑ Caruso, R.C., et al., Electroretinographic findings in the mucopolysaccharidoses. Ophthalmology, 1986. 93(12): p. 1612-6.
- ↑ Delleman, J.W. and P.T. de Jong, Pigment epithelial pattern dystrophy: a peripheral type. Br J Ophthalmol, 1985. 69(10): p. 754-7.
- ↑ 26.0 26.1 Alden, T.D., et al., Surgical management of neurological manifestations of mucopolysaccharidosis disorders. Mol Genet Metab, 2017. 122S: p. 41-48.
- ↑ Dalla Corte, A., et al., Hydrocephalus and mucopolysaccharidoses: what do we know and what do we not know? Childs Nerv Syst, 2017. 33(7): p. 1073-1080.
- ↑ 28.0 28.1 28.2 Fahnehjelm, K.T., et al., Ocular findings in four children with mucopolysaccharidosis I-Hurler (MPS I-H) treated early with haematopoietic stem cell transplantation. Acta Ophthalmol Scand, 2006. 84(6): p. 781-5.
- ↑ Pitz, S., et al., Ocular changes in patients with mucopolysaccharidosis I receiving enzyme replacement therapy: a 4-year experience. Arch Ophthalmol, 2007. 125(10): p. 1353-6.
- ↑ Schumacher, R.G., et al., Sonographic ocular findings in patients with mucopolysaccharidoses I, II and VI. Pediatr Radiol, 2008. 38(5): p. 543-50.
- ↑ Fahnehjelm, K.T., A.L. Tornquist, and J. Winiarski, Ocular axial length and corneal refraction in children with mucopolysaccharidosis (MPS I-Hurler). Acta Ophthalmol, 2012. 90(3): p. 287-90.
- ↑ 32.0 32.1 32.2 Ohden, K.L., et al., Outcomes of keratoplasty in the mucopolysaccharidoses: an international perspective. Br J Ophthalmol, 2017. 101(7): p. 909-912.
- ↑ Bothun, E.D., et al., Outcome of penetrating keratoplasty for mucopolysaccharidoses. Arch Ophthalmol, 2011. 129(2): p. 138-44.
- ↑ Harding, S.A., et al., Indications and outcomes of deep anterior lamellar keratoplasty in children. Ophthalmology, 2010. 117(11): p. 2191-5.
- ↑ Aliabadi, H., et al., Clinical outcome of cerebrospinal fluid shunting for communicating hydrocephalus in mucopolysaccharidoses I, II, and III: a retrospective analysis of 13 patients. Neurosurgery, 2010. 67(6): p. 1476-81; discussion 1481-2.
- ↑ 36.0 36.1 Neto, A.R., et al., Hydrocephalus in mucopolysaccharidosis type VI successfully treated with endoscopic third ventriculostomy. J Neurosurg Pediatr, 2013. 11(3): p. 327-30.
- ↑ Hendriksz, C.J., et al., Health-related quality of life in mucopolysaccharidosis: looking beyond biomedical issues. Orphanet J Rare Dis, 2016. 11(1): p. 119.
- ↑ Bergwerk, K.L., Y.S. Rabinowitz, and R.E. Falk, Quality of life related to visual function in three young adults with mucopolysaccharidoses. ScientificWorldJournal, 2003. 3: p. 922-9.
- ↑ Enoch, J., et al., Evaluating Whether Sight Is the Most Valued Sense. JAMA Ophthalmol, 2019. 137(11): p. 1317-1320.