Junctional Scotoma and Junctional Scotoma of Traquair
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Junctional scotoma and the junctional scotoma of Traquair are visual field defects that arise from damage to the junction of the optic nerve and the optic chiasm. Sellar masses including pituitary tumors are the most common cause of these visual field defects. Ophthalmologic findings include ipsilateral central scotoma and typically a contralateral superior temporal visual field defect (junctional scotoma, JS) or a monocular hemianopic field defect, which can be nasal or temporal known as junctional scotoma of Traquair JST.[1][2]
Disease Entity
Anatomy
The optic chiasm is formed by the union of the two optic nerves. At the junction of the optic nerve with the chiasm, 53% of the fibers from the nasal hemiretina of each eye will cross over while the fibers from the temporal hemiretina (47%) of each eye remain uncrossed. The optic chiasm has a transverse length of 12 - 18 mm, an anteroposterior width of 8 mm, and a height of 4 mm. It lies over the sella turcica and the pituitary gland. The optic chiasm is in direct contact with cerebrospinal fluid in the subarachnoid space anteriorly and forms the floor of the third ventricle posteriorly.[3]
Etiology
Lesions that produce a junctional scotoma or a junctional scotoma of Traquair are typically extrinsic compressive mass lesions at the junction of the optic nerve and the chiasm. The most common causes include: [1]
- Pituitary adenoma ( most common cause)
- Sphenoid wing meningioma
- Craniopharyngioma
- Aneurysms of the internal carotid or the anterior communicating artery
Other causes include demyelinating, infectious, inflammatory, infiltrative, traumatic, and other etiologies which can occur in this location. A rapid progressive junctional scotoma should raise alarm to a rapidly growing vascular pathology such as pituitary apoplexy or internal carotid artery aneurysm.
Pathology
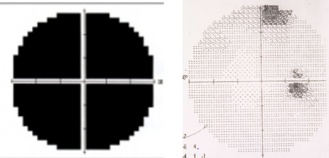
The junctional scotoma classically presents as a central scotoma (or other optic nerve type of visual field defect) in one eye as well as a superior temporal visual field defect respecting the vertical meridian (or a temporal visual field defect respecting the vertical midline) in the other eye. The lesion involves the ipsilateral optic nerve at the junction and also typically the contralateral crossing inferior nasal retinal fibers (producing the superotemporal visual field loss in the fellow eye). In the junctional scotoma of Traquir, the lesion , also at the junction of the optic nerve and chiasm, will cause a temporal or rarely a nasal visual field defect respecting the vertical midline ipsilateral to the lesion depending on which fibers get involved.
Diagnosis
History
Patients with either a junctional scotoma or junctional scotoma of Traquair complain of ipsilesional visual loss. Despite a superior temporal visual field defect in the contralesional eye in the typical junctional scotoma, the patient may be asymptomatic in that eye. Often these patients also present with endocrine abnormalities and headaches due to either mechanical compression of nervous structures or hormonal dysregulation from a pituitary macroadenoma for instance.
Physical examination
Patients may present with varying levels of decreased visual acuity (in addition to the visual field defects above). There will be an ipsilesional relative afferent pupillary defect (RAPD). Band type optic atrophy may be seen on fundus exam in cases which involve nasal fibers. In the junctional scotoma the band atrophy is in the contralesional eye (superotemporal visual field loss) and in the junctional scotoma of Traquair will be in the ipsilesional eye if the defect is monocular and a temporal hemianopsia is present. In the nasal hemianopic variety (rare) of the junctional scotoma of Traquair the optic atrophy will be more likely in the hour glass (temporal fiber atrophy) and not the band configuration (nasal fiber atrophy). Figure 1 shows the junctional scotoma of Traquair and Figure 2 shows the junctional scotoma. It is important to note, that attention to the asymptomatic eye should always be meticulous as failing to do a confrontation visual field testing may allow for missing a junctional scotoma, keeping in mind the confrontation fields are only useful when the visual field defect is picked up.
Diagnostic procedures
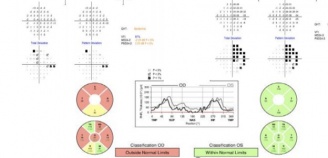
Formal visual field imaging is necessary to accurately diagnose patients with junctional visual field loss . Automated (e.g., Humphrey visual field testing) perimetry has been shown to be sensitive and specific in patients with visual field defects including junctional loss.[3] Optical Coherence Tomography (OCT) can also aid in identifying band atrophy when the damage involves nasal fibers, or the hour glass pattern of atrophy in cases affecting the temporal fibers. OCT of the macular ganglion cell layer can also document the findings and may predict final visual recovery following surgical correction of the underlying compressive cause.
Laboratory test
Laboratory studies may be considered in cases suspected to be from pituitary adenoma including hormone levels.[3] Infectious (e.g., syphilis, tuberculosis), inflammatory (e.g., sarcoid, granulomatous disease, vasculitis), infiltrative (e.g., lymphoproliferative), and demyelinating (e.g., multiple sclerosis, neuromyelitis optica[5], myelin oligodendrocytic glycoprotein) disorders should also be considered in the differential diagnosis especially if the neuroimaging does not disclose a compressive etiology or shows optic nerve/chiasmal enhancement after contrast administration.
Imaging
Computed tomography (CT) Scan can be used in traumatic cases to identify skull fractures in the frontal and anterior skull base, the orbital roof, the sphenoid bone. Damage to the sella turcica can also be visualized through this method.[6] CT scan is also a more rapid initial neuroimaging for acute cases but in general MRI is superior for imaging the junctional visual field loss. Magnetic resonance imaging (MRI) with contrast may show the chiasmal compressive lesion. High resolution MRI with contrast provides excellent imaging of the soft tissues and can be used to identify other causes such as enhancement, edema or herniation around the optic chiasm. MRI can also identify chiasmal contusion, hemorrhage, or tumor infiltration.[3] Cerebral Angiography is usually not necessary in junctional visual field loss but if initial neuroimaging suggests a vascular etiology then cerebral angiography may be helpful. The optic chiasm is surrounded by the anterior cerebral artery, the anterior communicating artery, the internal carotid artery, the middle cerebral artery, and the cavernous sinus. An aneurysm in these vascular structures can compress the adjacent nervous structures and a junctional visual field change can develop.[7]
Management
The treatment should be aimed at the underlying etiology but in general compressive lesions require surgical decompression. Corticosteroids may be used for inflammatory etiologies and antimicrobial therapy for infectious etiologies. Treatment differs according to the cause of the junctional visual field defect.
Prognosis
Visual prognosis depends on the cause and duration of symptoms. Analysis of the macular ganglion cell layer using OCT can help predict visual recovery in these cases.[8]
References
- ↑ Jump up to: 1.0 1.1 Borgman, Christopher J. “Atypical Junctional Scotoma Secondary to Optic Chiasm Atrophy: a Case Report.” Clinical and Experimental Optometry 102.6 (2019): 627–630. Web.
- ↑ Francesco Pellegrini, Alessandra Cuna, Daniele Cirone, Cristina Ciabattoni, Ettore Caruso, Emanuela Interlandi, Antonio Zappacosta. Clinical Reasoning: Wilbrand's Knee, Scotoma of Traquair, and Normal Tension Glaucoma. Case report neurol. 2022 Aug 30;14(2):341-347. doi: 10.1159/000525799. eCollection 2022 May-Aug.PMID: 36160657 PMCID: PMC9459516DOI: 10.1159/000525799
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Foroozan, Rod. “Chiasmal Syndromes.” Current Opinion in Ophthalmology 14.6 (2003): 325–331. Web.
- ↑ Shruthi Harish Bindiganavile 1, Nita Bhat, Ore-Ofeoluwatomi Adesina, Andrew G Lee. Optical Coherence Tomography Findings in the Junctional Scotoma of Traquair.2021 Mar 1;41(1):e111-e113. doi: 10.1097/WNO.0000000000000972.
- ↑ Alexander M Warwick, Sidney M Gospe, John J Chen. At this junction…Surv Ophthalmol 2022 Nov-Dec;67(6):1711-1716.DOI: 10.1016/j.survophthal.2021.08.001. PMID: 34364902
- ↑ Loganathan Vellayan Mookan, Philip A. Thomas, and Ankit Anil Harwani. “Traumatic Chiasmal Syndrome: A Meta-Analysis.” American Journal of Ophthalmology Case Reports 9.C (2018): 119–123. Web.
- ↑ Shin, Woo-Jae et al. “Junctional Scotoma in Giant Cerebral Aneurysm.” Korean journal of ophthalmology : KJO 16.2 (2002): 124–129. Web.
- ↑ Yoo YJ, Hwang JM, Yang HK, Joo JD, Kim YH, Kim CY. Prognostic value of macular ganglion cell layer thickness for visual outcome in parasellar tumors [published online ahead of print, 2020 Apr 6]. J Neurol Sci. 2020;414:116823. doi:10.1016/j.jns.2020.116823