All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Trabeculectomy has long been considered the gold standard in the surgical treatment of glaucoma due to its efficacy in lowering intraocular pressure (IOP). However, with the IOP-lowering capacity of trabeculectomy, comes risks for short- and long-term complications, including bleb leaks, infection, and over-filtration leading to choroidal effusion, hemorrhage, accelerated cataract progression, and hypotony. A number of glaucoma surgical devices and techniques have been developed in the last several decades, however few, if any, have been able to reliably replicate the IOP-lowering-effect of the trabeculectomy, without the complication risk.
Non-penetrating deep sclerectomy (NPDS) is a procedure which was first described in 1989, and has been shown to offer a similar, albeit by some accounts, slightly inferior, IOP-lowering effect with less risk of complication compared to trabeculectomy.[1] The earliest reports of non-penetrating filtering glaucoma surgery came from Kasnov and Walker in 1964, describing the sinusotomy.[2] The sinusotomy is a non-penetrating surgery which involves opening Schlemm’s canal, and excising a block of the canal’s outer wall. Non-penetrating procedures were further refined over the next several decades and in 1989, Kozlov and Fyodorov described the deep sclerectomy.[3] They detailed a surgery in which a flap of deep sclera was removed, Schlemm’s canal was deroofed, and the underlying juxtacanalicular trabecular meshwork was removed, forming a scleral space through which aqueous humor would drain. By removing the juxtacanalicular meshwork, NPDS effectively targets the site of maximal outflow resistance, thereby increasing aqueous humor outflow and lowering IOP. By not penetrating full thickness through the trabecular meshwork into the anterior chamber, NPDS was thought to offer more reliability and safety than trabeculectomy. However, NPDS has proven to be a technically difficult procedure with a long learning curve, and a high rate of conversion to trabeculectomy particularly in the hands of inexperienced surgeons.[4]
Today NPDS is considered a clinically reasonable option for many types and severities of glaucoma, and while rarely done in the United States, has become the preferred surgical technique for treating glaucoma in many European countries.[5]
Indications and Pre-operative Considerations
NPDS is indicated for the treatment of primary and secondary open angle glaucoma. Patients who have uncontrolled IOP despite maximally tolerated medical therapy (MTMT), laser, and/or incisional surgery, are often good candidates for this procedure. In fact, NPDS may be the best first-line surgical option in a number of clinical scenarios, based upon results published in the literature. Specifically, high-risk eyes or monocular patients who require a surgical option with a lower risk of complication[5], and in eyes which have already undergone a prior successful NPDS in the fellow eye.[6] Patients with uveitic glaucoma also benefit from NPDS, likely due to the decreased inflammation produced compared to penetrating procedures.[7][8] Eyes with long axial lengths are at a higher risk of hypotony after penetrating filtering surgeries such as trabeculectomy, and for this reason, should be considered for NPDS as well.[9] Patients with congenital glaucoma have also been shown to benefit from NPDS.[10]
An important patient factor that should be considered prior to NPDS is the ability to adhere to post-operative follow-up appointments and topical medication regimens. Like after a trabeculectomy, postoperative management focuses on early detection and treatment of infection, inflammation, and over-filtration. Close monitoring and prompt complication management increase the success of the surgery.[11]
Contraindications
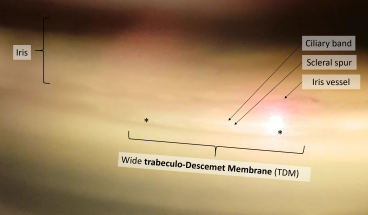
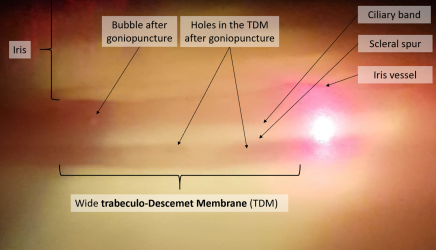
The contraindications to NPDS include non-functioning, closed, and narrow-angles, as this pathology prevents or limits aqueous humor outflow through the trabeculo-Descemet membrane (TDM), which NPDS relies on to have an effect.
Absolute contraindications:[12]
- Primary angle-closure glaucoma
- Secondary angle-closure glaucoma
- Neovascular glaucoma
Relative contraindications:[12]
- Narrow-angle glaucoma (combined NPDR and cataract surgery may be performed as removing the lens will allow a partial opening of the iridocorneal angle)
- Traumatic glaucoma (NPDS may still be performed if the area of angle recession or trabecular damage is small and lies outside the surgery area)
- Glaucoma secondary to increased episcleral venous pressure
Surgical technique
- Exposure: A corneal traction suture is placed superiorly, and the globe is tilted down.
- Conjunctival dissection: The conjunctival dissection can be limbal-based or fornix-based. A fornix-based conjunctival dissection involves opening the conjunctiva and Tenon’s at the limbus and deeply dissecting posteriorly under Tenon’s capsule. In a limbal-based approach, the conjunctiva and Tenon’s are opened posteriorly, about 10 mm from the limbus, and an anterior dissection under Tenon’s is performed, toward the limbus. Both techniques have equivalent IOP lowering effects,[13][14] and have their own advantages and disadvantages.
- Preparation of the sclera and application of antimetabolite: The remains of the episclera are removed and gentle diathermy/electrocautery hemostasis is performed, if necessary. Antimetabolite can then be applied to reduce the risk of postoperative fibrosis. The most commonly used antimetabolites are 5-fluorouracil and mitomycin C. The choice of agent depends on the presence of risk factors for scarring: <50 years, Afro-Caribbean / Hispanic patient, known inflammatory disease, chronic use of topical medication, aphakia, recent eye surgery (<3 months), previous conjunctival surgery, previously failed filtering surgery, and neovascular glaucoma.[15] The application of the antimetabolite agent to the sclera should be as wide and posterior as possible to promote a posterior extension of the filtration bleb and to minimize the risk of obtaining small, localized, cystic blebs.[16][17]
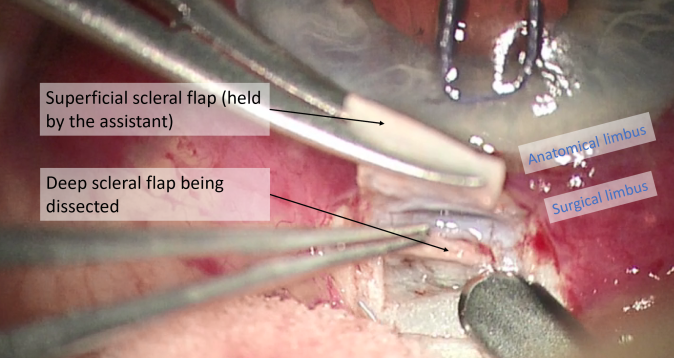
- Superficial scleral flap: The superficial scleral flap represents the roof of the decompression chamber. The flap can be triangular, rectangular, or trapezoidal, and should be one third to one half scleral thickness, 3-5 mm in length. A 15° blade is helpful in creating the initial flap edges, while a beveled blade is often used for flap dissection.
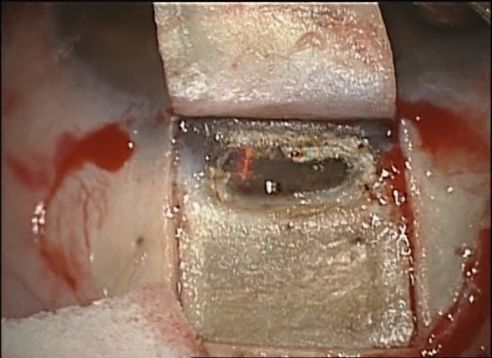
- Deep scleral flap: Two methods can be used for the dissection of the deep scleral flap. Most often, a rectangular (or triangular) deep scleral flap is dissected starting posteriorly in a pre-choroidal deep scleral plane. This depth is maintained as the dissection plane advances anteriorly, to identify the scleral spur and the canal of Schlemm. Alternatively, and less commonly, the paralimbic area can be directly approached by a radial incision in the area of the trabecular meshwork and the scleral spur. Regardless of approach, dissection along the scleral spur will open Schlemm’s canal, and excision of the deep flap is performed at its base.
- Peeling of the internal wall of the Schlemm's canal: The cribriform trabeculum and Schlemm's canal are peeled with capsulorhexis or Bonn forceps. At this step, it is important to avoid compression of the eye which can lead to inadvertent penetration into the anterior chamber.
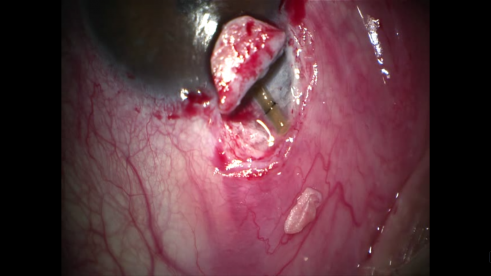
- Repositioning of the superficial scleral flap: Though their use is not critical, non-absorbable sutures can be used to position the superficial scleral flap. Unlike in trabeculectomy, these sutures are not to titrate aqueous humor outflow, but rather to facilitate a smooth surface for healing.
- Conjunctival sutures: Like in trabeculectomy, the closure of the conjunctiva must be watertight. Vicryl suture on a round needle is typically used. The presence of the Tenons at the closure site is thought to be useful for watertight sealing.
Additional surgical steps:
- CO2 Laser-Assisted Sclerectomy Surgery (CLASS): The carbon dioxide (CO2) laser-assisted sclerectomy surgery (CLASS) offers a safe and simple approach to scleral dissection, with a shorter learning curve and surgical time, compared to traditional NPDS.[18] The CO2 laser scanning system (IOPtiMate, OT-135 and OT-136 models; IOPtima Ltd, Caesarea, Israel) is highly effective in ablating dry tissue, and allows the surgeon to control the thinning of scleral tissue, leading to more accurate processing of Schlemm’s canal and the TDM window. The CLASS approach is utilized after constructing a partial thickness scleral flap, as is done in traditional NPDS.[19] The scanning CO2 laser system (IOPtiMate) is then used to create the deep scleral flap and unroof Schlemm’s canal. The infrared CO2 laser is safe and self-regulated in that it is absorbed and blocked by aqueous solutions, preventing penetration into the eye.
- Installation of a drain/implant: Space-maintaining devices can be used to maintain the scleral space, post-operatively.[20] The implant can be added after the deep scleral flap has been created, and the internal wall of Schlemm’s canal has been excised. There are two groups of implants, absorbable and non-absorbable. T-flux® is a type of non-absorbable implant made of poly-Megma®, a highly hydrophilic acrylic synthetic substance. T-flux® is in the shape of a T, and the arms are inserted into the openings of Schlemm's canal.[21] More recently, a non-absorbable acrylic polymer implant called Esnoper®, has been developed.[22]This implant is a small tetrahedral plate designed to be placed on the scleral bed and contains several longitudinal channels to maximize aqueous humor drainage. The first absorbable implant to be commercialized was Aquaflow®, a cylindrical collagen implant that is placed in the scleral bed, triples in volume after hydration, and is degraded in 6-9 months.[23] Other space-maintaining materials, such as SK-gel® (a reticulated hyaluronic acid), or Healon GV (a viscoelastic agent) can be used to maintain the intrascleral lake.[22]
Pearls for success
- High magnification is imperative, in particular during the second flap creation, and unroofing of Schlemm's canal.
- The second/deep scleral flap should almost reach the choroid posteriorly to achieve sufficient depth to allow the identification of Schlemm's canal.
- The corneal traction suture is vital for good visualization, but it should be loosened during the deep flap dissection and Schlemm’s peeling to prevent compression of the eye.[12]
- After deep flap dissection, a paracentesis can be helpful to avoid penetration during peeling of the internal wall of Schlemm’s canal, as it will decrease bulging of the trabeculum.
- To peel the internal wall of Schlemm's canal, it is crucial to use gentle movements and avoid downward pressure, which might cause penetration.[12]
- If penetration occurs, a micro-iridectomy can be performed to prevent the iris from obstructing the TDM. It is also frequently possible to perform a rescue laser iridoplasty during the postoperative period.
Post operative Course
Postoperative follow-up
Close monitoring and prompt complication management increase the success of the surgery.[11] We suggest the following post-operative follow-up schedule:
- Day 0-1: to identify early complications such as hypotony and hemorrhage.[12][24]
- Day 4-8: to detect and manage early complications such as endophthalmitis, hyphema, encysted bleb, choroidal detachment, hypotony maculopathy, anterior uveitis, flat anterior chamber, bleb leak, epithelial defect. If complications arise, alternative management and more frequent follow-up visits should be arranged.
- Day 14, Month 1, Month 3, Month 6, Month 9, Month 12, etc.
Medical treatment:
Postoperative medical treatment aims to decrease the risk of infection and inflammation. There is no consensus regarding the ideal post-operative medication, but steroids are generally considered more effective than NSAIDs,[25] and high doses of corticosteroid with a slow taper seem to lead to fewer complications and better IOP stabilization.[26]
The authors propose the following postoperative regimen: combined dexamethasone-antibiotics (e.g., tobramycin + dexamethasone, six times a day for 1-2 weeks, then four times a day for another 2-4 weeks), tropicamide (at night for two days, to decrease inflammation), NSAIDs (two to three times a day for 15 days). The patient must be advised to refrain from physical activity, shield the eye, and present for care if pain or decreased vision occurs.
Complications
NPDS generally has a favorable safety profile. The range of complications are similar to those associated with filtering surgeries. Examples of such complications include: bleb leaks, infection, over-filtration leading to choroidal effusion and hemorrhage, accelerated cataract progression, and hypotony.[9]
The two most common intraoperative complications during NPDS are an inability to find Schlemm’s canal, and TDM perforation. An inability to find Schlemm's canal occurs due to an insufficiently deep dissection of the scleral canal, often due to fear of dissecting too deep into the choroid. In these instances, a third deep scleral flap can be created to achieve the correct plane needed to identify Schlemm’s canal. TDM perforation can occur relatively easily as this membrane is exceptionally fragile. While a small perforation without iris prolapse has no consequence, a large perforation may lead to iris incarceration/prolapse. A large perforation leading to iris prolapse requires either viscoelastic injection or a peripheral iridectomy, effectively leading to trabeculectomy conversion.[9][12]
Intraocular hemorrhage is a rare complication of NPDS, as the IOP reduction is slower and more predictable compared to penetrating surgeries.[27] Hypotony is very common in the early postoperative period and often does not need to be treated if not associated with a shallow anterior chamber or maculopathy. In fact, Shaaraway showed that IOP < 5mmHg in the first postoperative day was a predictive factor for a successful surgery.[12]
Increased IOP in the early post-operative period can occur due to robust inflammatory response, retained viscoelastic, insufficient TDM dissection, blockage of the scleral bed or bleb by corneal or scleral tissue, or iris incarceration. Insufficient TDM dissection can be treated with goniopuncture, and blockage of the scleral bed can be treated with surgical revision. As previously mentioned, iris incarceration can be addressed with viscoelastic injection, miotics, iridotomy, iridoplasty, or with surgical revision. Late increased IOP can occur due to a less permeable TDM, bleb fibrosis, or steroid response. A progressive rise in IOP occurs in up to 60% of patients during the first year after NPDS,[9][12] and if due to reduced TDM permeability, can be managed with goniopuncture.
Descemet membrane detachment can arise weeks or months after NPDS and manifests with deep corneal bullae and stromal opacity. The cause is typically a rise in IOP due to an encysted bleb, trauma, or excessive eye massage. Treatment consists of draining the stagnant fluid and addressing the underlying cause. A period of observation is recommended before attempting a descemetopexy with intracameral air.[28]
Blebitis and endophthalmitis are extremely rare complications after NPDS, with only one case reported in the literature.[29] Hypotony is also very uncommon, with one retrospective cohort finding the rate of postoperative hypotony-related complications to be 5.6%, five years after NPDS.[30]
Goniopuncture
After NPDS, the primary determinant of aqueous outflow is the TDM. Resistance to aqueous flow through the TDM may increase over time, inducing a rise in IOP. When this occurs, laser goniopuncture (LGP) can be performed which creates a full-thickness hole in the TDM, decreasing outflow resistance of aqueous humor into the sub-conjunctival space.[31]
Before performing LGP, however, it is important to rule out other causes of post-operative IOP increase, specifically those related to bleb function (i.e., encysted or encapsulated bleb, subconjunctival fibrosis), and TDM patency (i.e., iris incarceration). Goniopuncture is often discouraged within the first three months after NPDS, because of the risk of hypotony as well as IOP increase due to iris incarceration.[31] Penaud et al. reported more iris incarceration after LGP in the first three months after NPDS (25.4%), compared to after three months (11.4%).[32] Di Matteo et al. reported similar results.[33]
Laser goniopuncture procedure and follow-up:
- Apply pilocarpine 2% and topical anesthesia
- Prophylactic argon iridoplasty or peripheral iridotomy can be performed if not already done previously,[32][33] especially if there is a convex peripheral iris or shallow peripheral anterior chamber.
- The angle is visualized using a single-mirror contact-lens with coupling gel
- The Nd:Yag laser (Q-switched mode, single-shot) is used. The guide beam is aimed at the anterior edge of the TDM, on either side of the scleral window, to reduce the risk of iris incarceration. The initial energy level should be around 2mJ, titrated to the appearance of micro blebs, and 1-2 micro-perforations occur. Perforation may be difficult to confirm if the remained trabeculum is not pigmented. In these cases, slight pressure can be applied to the eye to observe the movement of micro-blebs through the holes in TDM.[34]
- After LGP, pilocarpine 2% is instilled three times a day for 7-14 days, sometimes in combination with steroids or NSAIDs twice a day, for 1-4 weeks. No evidence to date exists to support the use of either, or both, anti-inflammatory medications after goniopuncture.
- The patient should follow-up in 1-3 weeks for IOP check and repeat gonioscopy. LGP can be repeated if no punctures are visible in the TDM during follow-up, or the IOP remains high.
Complications of goniopuncture:
The occurrence of severe complications after LGP is rare. The most frequent complications are iris prolapse/incarceration, which occurs in up to 17.6% of cases, and bleeding. Di Matteo et al. observed less iris incarceration after LGP if prophylactic iridoplasty and/or iridotomy were previously performed.[33] Therefore, iridoplasty should be performed prior to goniopuncture especially in eyes with convex peripheral irises, shallow peripheral anterior chambers, and high pre-treatment IOP.
The application of IOP lowering medication before goniopuncture decreases the risk of iris incarceration and hemorrhage.[34] In the case of iris prolapse, iris retraction can be attempted with pilocarpine, and/or repeated argon laser applied to the peripheral iris. If these interventions fail, surgical revision may be required for iridectomy and iris repositioning. If hemorrhage occurs during LGP, the procedure should be discontinued, and pressure should be applied to the eye. In case of shallow or flat anterior chamber after LGP, cycloplegia should be added, and if persistent, the anterior chamber should be reformed with viscoelastic.[34] Other rare complications after LGP include IOP spike, iritis, peripheral synechia formation, blebitis, and hypotony.
Outcomes
After NPDS, LGP is performed in 35.7-67% of cases, usually within the first year after surgery.[31][34][33] Harju et al. reported a rate of up to 100% of LGP after NPDS, over a follow up period of up to nine years.[35] LGP is associated with a significant and persistent reduction of IOP, ranging from 27-58%.[35][31][34]
Results
NPDS is a reasonable option for many primary and secondary open-angle glaucoma patients at any point in their disease course. Though it has a long learning curve and requires greater operative time, NPDS carries a lower complication rate, and provides a a similar, albeit slightly inferior, IOP-lowering effect compared to trabeculectomy.[9][27][5]
NPDS with collagen implant has been reported to bring satisfactory results over a three year follow-up, with a reported mean decrease in IOP to 12.4 ± 3.9 mmHg (55.4% reduction) at three months and 13.0 ± 3.8 mmHg (53.2% reduction) at three years of follow-up.[36] One study found the complete success rate (IOP ≤ 21 mmHg without medication) of NPDS with collagen implant to be 62% and 57%, at five and seven years, respectively.[37] In eyes that have failed a previous trabeculectomy, NPDS with mitomycin-C has shown a 65% complete success rate (IOP <21mmHg under no topical medication).[38] Khairy et al. confirmed that NPDS reduced IOP and minimized the risk of post-operative complications compared to trabeculectomy, however, also found that NPDS failed to maintain a low IOP after long-term follow-up (18.8% success at 30 months).[39]
A meta-analysis evaluating the efficacy and safety of non-penetrating surgical (NPS) procedures (deep sclerectomy, viscocanalostomy, and canaloplasty) versus trabeculectomy showed that trabeculectomy was slightly more effective at reducing IOP than NPS at 6 and 12 months post-operatively (−2.15 mm Hg and −2.22 mm Hg, respectively). One study comparing NPDS performed in one eye and trabeculectomy in the other, found the complete success at two years to be 45% in the trabeculectomy eyes, and 40% in the NPDS eyes.[12][1] In a meta-analysis, however, no significant difference was found with regard to the number of anti-glaucoma medications between patients who underwent trabeculectomy and those who underwent NDPS.[40] Meta-analyses assessing the risk of complications of trabeculectomy and NPDS have found that trabeculectomy is associated with a higher rate of complications compared to NPDS.[40][41]
References
- ↑ Jump up to: 1.0 1.1 Ambresin A, Shaarawy T, Mermoud A. Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. Journal of glaucoma 2002;11(3):214-20. doi: 10.1097/00061198-200206000-00009 [published Online First: 2002/07/26].
- ↑ Krasnov MM. [Sinusotomy in Glaucoma]. Vestnik oftalmologii 1964;77:37-41. [published Online First: 1964/03/01].
- ↑ SN Fyodorov. Non penetrating deep sclerectomy in open-angle glaucoma (Russian). . Eye Microsurg 1989;1:52-5.
- ↑ Varga, Zsolt, and Tarek Shaarawy. 2009. “Deep Sclerectomy: Safety and Efficacy.” Middle East African Journal of Ophthalmology 16 (3): 123–26.
- ↑ Jump up to: 5.0 5.1 5.2 Setten Gv. Survival of the best? - Why some surgical techniques such as deep sclerectomy 'fail' [published online ahead of print, 2020 Mar 12]. Acta Ophthalmol. 2020;10.1111/aos.14383. doi:10.1111/aos.14383.
- ↑ Gillmann, Kevin, Enrico Meduri, Archibald Paillard, Giorgio E. Bravetti, Harsha L. Rao, André Mermoud, and Kaweh Mansouri. 2022. “Bilateral Nonpenetrating Deep Sclerectomy: Difference in Outcomes between First- and Second-Operated Eyes at 24 Months.” Journal of Glaucoma 31 (2): 109–15.
- ↑ Mendrinos, Efstratios, André Mermoud, and Tarek Shaarawy. 2008. “Nonpenetrating Glaucoma Surgery.” Survey of Ophthalmology 53 (6): 592–630.
- ↑ Anand N. Deep sclerectomy with mitomycin C for glaucoma secondary to uveitis. European journal of ophthalmology 2011;21(6):708-14. doi: 10.5301/EJO.2011.6487 [published Online First: 2011/03/30].
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Carassa, RG. (2012) Non-penetrating surgery. GL Spaeth (Ed.) In Ophthalmic Surgery: Principles and Practice; fourth edition, Chapter 38. 256-263. Elsevier Inc.
- ↑ Malik R, AlDarrab A, Edward DP. Contemporary management of refractory pediatric glaucoma. Current opinion in ophthalmology 2020;31(2):123-31. doi: 10.1097/ICU.0000000000000642 [published Online First: 2020/01/03].
- ↑ Jump up to: 11.0 11.1 Jonescu-Cuypers CP1, Seitz B. Postoperative complications and management of filtration surgery]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen; DOI: 10.1007/s00347-009-2071-5
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Tarek Shaarawy TD, Shibal Bhartyia. ISGS Textbook of Glaucoma Surgery. Jaypee Brothers Medical Publishers 2014:181-90.
- ↑ Kozobolis VP, Siganos CS, Christodoulakis EV, et al. Two-site phacotrabeculectomy with intraoperative mitomycin-C: fornix- versus limbus-based conjunctival opening in fellow eyes. J Cataract Refract Surg 2002;28(10):1758-62. doi: 10.1016/s0886-3350(02)01270-1 [published Online First: 2002/10/22].
- ↑ Solus JF, Jampel HD, Tracey PA, et al. Comparison of limbus-based and fornix-based trabeculectomy: success, bleb-related complications, and bleb morphology. Ophthalmology 2012;119(4):703-11. doi: 10.1016/j.ophtha.2011.09.046 [published Online First: 2012/01/10].
- ↑ Melo AB, Spaeth GL. A new, safer method of applying antimetabolites during glaucoma filtering surgery. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye 2010;41(3):383-5. doi: 10.3928/15428877-20100430-14 [published Online First: 2010/05/29].
- ↑ Khaw PT, Chang L, Wong TT, et al. Modulation of wound healing after glaucoma surgery. Current opinion in ophthalmology 2001;12(2):143-8. doi: 10.1097/00055735-200104000-00011 [published Online First: 2001/02/27].
- ↑ Khaw PT, Chiang M, Shah P, et al. Enhanced Trabeculectomy: The Moorfields Safer Surgery System. Dev Ophthalmol 2017;59:15-35. doi: 10.1159/000458483 [published Online First: 2017/04/27].
- ↑ Yu, Xiaojiao; Chen, Chunlin; Sun, Min; Dong, Denghao; Zhang, Shuoji; Liu, Pei; Yuan, Rongdi; Ye, Jian. CO2 Laser assisted Deep Sclerectomy Combined With Phacoemulsification in Patients With Primary Open-angle Glaucoma and Cataract. Journal of Glaucoma: October 2018, Volume 27, Issue 10, p 906-909
- ↑ Villavicencio, Quezada Baltodano, Ramírez Jiménez, Méndez, Ponte-Dávila Comparative Clinical Results of Phacoemulsification Combined with CO2 Laser-Assisted Sclerectomy vs. Phacoemulsification Combined with Trabeculectomy in Patients with Open-Angle Glaucoma. J Clin Exp Opthamol 2018, 9:5
- ↑ Elhofi, Abdelhamid, and Hany Ahmed Helaly. 2018. “Outcome of Primary Nonpenetrating Deep Sclerectomy in Patients with Steroid-Induced Glaucoma.” Journal of Ophthalmology 2018: 1–7.
- ↑ Wiermann A, Zeitz O, Jochim E, et al. [A comparison between absorbable and non-resorbable scleral implants in deep sclerectomy (T-Flux and SK-Gel)]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 2007;104(5):409-14. doi: 10.1007/s00347-007-1520-2 [published Online First: 2007/04/05].
- ↑ Jump up to: 22.0 22.1 Loscos-Arenas J, Parera-Arranz A, Romera-Romera P, et al. Deep Sclerectomy With a New Nonabsorbable Uveoscleral Implant (Esnoper-Clip): 1-Year Outcomes. Journal of glaucoma 2015;24(6):421-5. doi: 10.1097/IJG.0000000000000253 [published Online First: 2015/04/04].
- ↑ Shaarawy, Tarek, Kaweh Mansouri, Corinne Schnyder, Emile Ravinet, Farid Achache, and André Mermoud. 2004. “Long-Term Results of Deep Sclerectomy with Collagen Implant.” Journal of Cataract and Refractive Surgery 30 (6): 1225–31.
- ↑ Kyari F AM. The basics of good postoperative care after glaucoma surgery. Community Eye Health 2016;29:29–31.
- ↑ Almatlouh A, Bach-Holm D, Kessel L. Steroids and nonsteroidal anti-inflammatory drugs in the postoperative regime after trabeculectomy - which provides the better outcome? A systematic review and meta-analysis. Acta ophthalmologica 2019;97(2):146-57. doi: 10.1111/aos.13919 [published Online First: 2018/09/23].
- ↑ Araujo SV, Spaeth GL, Roth SM, et al. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology 1995;102(12):1753-9. doi: 10.1016/s0161-6420(95)30797-x [published Online First: 1995/12/01].
- ↑ Jump up to: 27.0 27.1 Roy S, Mermoud A. Deep Sclerectomy. Dev Ophthalmol 2017;59:36-42. doi: 10.1159/000458484 [published Online First: 2017/04/27].
- ↑ Ravinet E, Tritten JJ, Roy S, et al. Descemet membrane detachment after non-penetrating filtering surgery. Journal of glaucoma 2002;11(3):244-52. doi: 10.1097/00061198-200206000-00014 [published Online First: 2002/07/26].
- ↑ Chiselita D, Danielescu C. [Postoperative endophthalmitis after non-pentrating deep sclerectomy]. Oftalmologia 2010;54(1):36-40. [published Online First: 2010/06/15].
- ↑ Rabiolo, Alessandro, Duncan Leadbetter, and Nitin Anand. 2021. “Hypotony-Associated Complications after Deep Sclerectomy.” Journal of Glaucoma Publish Ahead of Print (7): e314–26.
- ↑ Jump up to: 31.0 31.1 31.2 31.3 Holmes D, Hui MMP, Clement C. Comparison of early versus late laser goniopuncture following deep sclerectomy for the management of open-angle glaucoma. The British journal of ophthalmology 2020 doi: 10.1136/bjophthalmol-2019-315392 [published Online First: 2020/02/07].
- ↑ Jump up to: 32.0 32.1 Penaud B, Leleu I, Laplace O, et al. Outcomes of Laser Goniopuncture Following Nonpenetrating Deep Sclerectomy With Mitomycin C: A Large Retrospective Cohort Study. Journal of glaucoma 2019;28(1):51-55. doi: 10.1097/IJG.0000000000001104 [published Online First: 2018/10/10].
- ↑ Jump up to: 33.0 33.1 33.2 33.3 Di Matteo F, Bettin P, Fiori M, et al. Nd:Yag laser goniopuncture for deep sclerectomy: efficacy and outcomes. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2016;254(3):535-9. doi: 10.1007/s00417-016-3271-8 [published Online First: 2016/02/03].
- ↑ Jump up to: 34.0 34.1 34.2 34.3 34.4 Tam DY, Barnebey HS, Ahmed, II. Nd: YAG laser goniopuncture: indications and procedure. Journal of glaucoma 2013;22(8):620-5. doi: 10.1097/IJG.0b013e31824d512a [published Online First: 2013/05/21].
- ↑ Jump up to: 35.0 35.1 Harju M, Suominen S, Allinen P, et al. Long-term results of deep sclerectomy in normal-tension glaucoma. Acta ophthalmologica 2018;96(2):154-60. doi: 10.1111/aos.13529 [published Online First: 2017/08/24].
- ↑ Karlen ME, Sanchez E, Schnyder CC, et al. Deep sclerectomy with collagen implant: medium term results. The British journal of ophthalmology 1999;83(1):6-11. doi: 10.1136/bjo.83.1.6 [published Online First: 1999/04/21].
- ↑ Shaarawy, T., M. Karlen, C. Schnyder, F. Achache, E. Sanchez, and A. Mermoud. 2001. “Five-Year Results of Deep Sclerectomy with Collagen Implant.” Journal of Cataract and Refractive Surgery 27 (11): 1770–78.
- ↑ Rebolleda G, Munoz-Negrete FJ. Deep sclerectomy with mitomycin C in failed trabeculectomy. Eye (London, England) 2007;21(1):23-8. doi: 10.1038/sj.eye.6702183 [published Online First: 2005/11/29].
- ↑ Khairy HA, Green FD, Nassar MK, et al. Control of intraocular pressure after deep sclerectomy. Eye (London, England) 2006;20(3):336-40. doi: 10.1038/sj.eye.6701878 [published Online First: 2005/04/16].
- ↑ Jump up to: 40.0 40.1 Gabai A, Cimarosti R, Battistella C, et al. Efficacy and Safety of Trabeculectomy Versus Nonpenetrating Surgeries in Open-angle Glaucoma: A Meta-analysis. Journal of glaucoma 2019;28(9):823-33. doi: 10.1097/IJG.0000000000001323 [published Online First: 2019/07/25].
- ↑ Eldaly MA, Bunce C, Elsheikha OZ, et al. Non-penetrating filtration surgery versus trabeculectomy for open-angle glaucoma. Cochrane Database Syst Rev 2014(2):CD007059. doi: 10.1002/14651858.CD007059.pub2 [published Online First: 2014/02/18].